H C C H Lewis Structure
arrobajuarez
Nov 09, 2025 · 10 min read
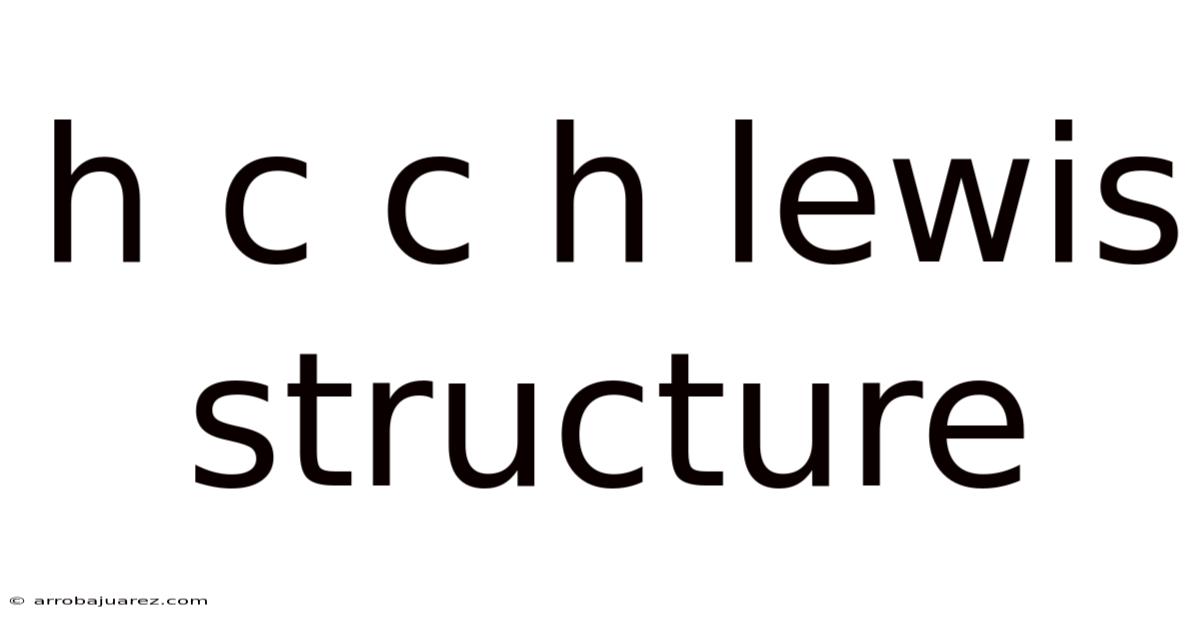
Table of Contents
The HCCH Lewis structure, representing the molecule ethyne (acetylene), is a fundamental concept in understanding chemical bonding and molecular structure. This simple yet crucial molecule serves as an excellent example for illustrating the principles of covalent bonding, sigma (σ) and pi (π) bonds, and the overall geometry of organic compounds.
Understanding the Basics: What is a Lewis Structure?
A Lewis structure, also known as an electron dot diagram, is a visual representation of the bonding between atoms in a molecule, as well as any lone pairs of electrons that may exist. Developed by Gilbert N. Lewis, these diagrams help us understand how electrons are arranged in a molecule, which in turn dictates its chemical properties and reactivity.
Key components of a Lewis structure include:
- Atomic symbols: Representing each atom in the molecule (e.g., H for hydrogen, C for carbon).
- Lines: Representing covalent bonds, where a single line indicates a single bond (two shared electrons), a double line indicates a double bond (four shared electrons), and a triple line indicates a triple bond (six shared electrons).
- Dots: Representing non-bonding electrons, also known as lone pairs, that are not involved in bonding.
Why is the HCCH Lewis Structure Important?
The HCCH Lewis structure, or the Lewis structure for ethyne (acetylene), is particularly important for several reasons:
- Illustrates Multiple Bonds: Ethyne contains a triple bond between the two carbon atoms, showcasing the concept of multiple covalent bonds. This is a prime example for students learning about different types of bonds.
- Demonstrates Molecular Geometry: The linear geometry of ethyne, directly derived from its Lewis structure, is crucial for understanding its reactivity and how it interacts with other molecules.
- Foundation for Organic Chemistry: Ethyne is a fundamental building block in organic chemistry. Understanding its structure is essential for comprehending more complex organic molecules and reactions.
- Applications in Industry: Acetylene is widely used in industrial applications such as welding, cutting, and as a precursor in the synthesis of various polymers and organic compounds.
Constructing the HCCH Lewis Structure: A Step-by-Step Guide
Creating the Lewis structure for HCCH involves a series of logical steps to ensure that all atoms achieve a stable electron configuration, typically following the octet rule (or duet rule for hydrogen). Here’s a detailed guide:
1. Determine the Total Number of Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. To find the total number of valence electrons in HCCH:
- Hydrogen (H) has 1 valence electron, and there are two hydrogen atoms: 2 x 1 = 2 valence electrons.
- Carbon (C) has 4 valence electrons, and there are two carbon atoms: 2 x 4 = 8 valence electrons.
- Total valence electrons: 2 + 8 = 10 valence electrons.
2. Draw the Basic Molecular Skeleton
Connect the atoms with single bonds. In ethyne, the two carbon atoms are bonded to each other, and each carbon is bonded to a hydrogen atom. The basic skeleton looks like this:
H – C – C – H
This uses 3 single bonds, which accounts for 3 x 2 = 6 valence electrons.
3. Distribute the Remaining Electrons as Lone Pairs
We started with 10 valence electrons and have used 6, leaving 4 electrons to be distributed. Place these remaining electrons as lone pairs on the carbon atoms to satisfy the octet rule (each carbon needs 8 electrons around it). However, with only 4 electrons remaining, placing them as lone pairs on the carbon atoms won't fulfill the octet rule.
4. Convert Lone Pairs into Multiple Bonds
Since the carbon atoms do not have a full octet, we need to form multiple bonds. Move the lone pairs between the carbon atoms to create a triple bond:
H – C ≡ C – H
Now, each carbon atom has 8 electrons around it (1 from the single bond to hydrogen and 3 from the triple bond to the other carbon), fulfilling the octet rule. Hydrogen already satisfies its duet rule with one bond.
5. Final HCCH Lewis Structure
The final Lewis structure for HCCH (ethyne) is:
H – C ≡ C – H
In this structure:
- Each hydrogen atom is connected to a carbon atom with a single bond.
- The two carbon atoms are connected by a triple bond, consisting of one sigma (σ) bond and two pi (π) bonds.
- There are no lone pairs on any of the atoms.
Understanding Sigma (σ) and Pi (π) Bonds in HCCH
The triple bond in ethyne is composed of different types of covalent bonds: sigma (σ) and pi (π) bonds. Understanding these is crucial for grasping the molecule's stability and reactivity.
-
Sigma (σ) Bond: This is a type of covalent bond formed by the head-on overlap of atomic orbitals. It is the strongest type of covalent bond and is present in all single, double, and triple bonds. In ethyne, one of the three bonds between the carbon atoms is a sigma bond.
-
Pi (π) Bond: This is a type of covalent bond formed by the sideways overlap of p-orbitals. It is weaker than a sigma bond. Pi bonds are present in double and triple bonds. In ethyne, two of the three bonds between the carbon atoms are pi bonds.
Therefore, the triple bond in ethyne consists of one sigma (σ) bond and two pi (π) bonds. This arrangement contributes to the molecule's rigidity and high electron density between the carbon atoms.
Molecular Geometry of HCCH
The Lewis structure of HCCH directly informs its molecular geometry. According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, electron pairs (both bonding and non-bonding) around a central atom will arrange themselves to minimize repulsion.
In ethyne:
- Each carbon atom is bonded to two other atoms (one hydrogen and one carbon).
- There are no lone pairs on the carbon atoms.
This arrangement leads to a linear geometry around each carbon atom. The entire molecule is therefore linear, with a bond angle of 180 degrees between the atoms.
Hybridization in HCCH
Understanding hybridization is essential for a complete picture of the bonding in ethyne. Hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the formation of covalent bonds.
In ethyne, each carbon atom undergoes sp hybridization. This means that one s orbital and one p orbital on each carbon atom mix to form two sp hybrid orbitals. The remaining two p orbitals remain unhybridized.
- sp Hybrid Orbitals: These form the sigma (σ) bonds. One sp orbital on each carbon atom overlaps with the 1s orbital of a hydrogen atom, forming the C-H sigma bond. The other sp orbital on each carbon atom overlaps with the sp orbital of the other carbon atom, forming the C-C sigma bond.
- Unhybridized p Orbitals: These form the pi (π) bonds. Each carbon atom has two unhybridized p orbitals that are perpendicular to each other and to the axis of the sigma bonds. These p orbitals overlap sideways to form the two pi (π) bonds of the triple bond.
The sp hybridization explains the linear geometry of ethyne and the high electron density in the triple bond region.
Properties and Reactivity of Ethyne (Acetylene)
The unique structure of ethyne, as revealed by its Lewis structure, dictates its properties and reactivity:
- High Reactivity: The presence of the triple bond, particularly the two pi bonds, makes ethyne highly reactive. Pi bonds are weaker than sigma bonds and are more easily broken, allowing ethyne to undergo addition reactions readily.
- Acidity: The hydrogen atoms in ethyne are weakly acidic. This is due to the sp hybridization of the carbon atoms, which increases the s-character of the C-H bond, making the hydrogen atoms more positive and easier to remove.
- Combustibility: Ethyne is highly flammable and burns with a sooty flame. This is due to its high carbon-to-hydrogen ratio.
- Linear Geometry: The linear geometry of ethyne influences its physical properties, such as its boiling point and melting point.
Applications of Ethyne (Acetylene)
Ethyne (acetylene) has numerous industrial applications, primarily due to its high energy content and reactivity:
- Welding and Cutting: Acetylene is used in oxy-acetylene torches for welding and cutting metals. When burned with oxygen, it produces a very hot flame (over 3,300 °C), making it suitable for these applications.
- Chemical Synthesis: Acetylene is a crucial starting material in the synthesis of various organic compounds, including plastics, synthetic fibers, and pharmaceuticals. It can be converted into ethylene, vinyl chloride, and acrylonitrile, which are used to produce polyethylene, polyvinyl chloride (PVC), and acrylic fibers, respectively.
- Lighting: Historically, acetylene was used in lamps for lighting, particularly in mining and portable lighting systems.
- Polymer Production: Acetylene is used in the production of various polymers, including polyacetylene, which is a conductive polymer.
Common Mistakes When Drawing HCCH Lewis Structure
When drawing the Lewis structure for HCCH, students often make several common mistakes:
- Incorrect Number of Valence Electrons: Failing to correctly count the total number of valence electrons is a common error. Always double-check the number of valence electrons for each atom.
- Incorrect Placement of Bonds: Placing the bonds incorrectly, such as forming a double bond instead of a triple bond between the carbon atoms, will lead to an incorrect Lewis structure.
- Not Satisfying the Octet Rule: Forgetting to ensure that each carbon atom has a full octet of electrons is another common mistake. Remember to form multiple bonds as needed.
- Adding Lone Pairs Incorrectly: Adding lone pairs to atoms that do not need them or forgetting to add them when necessary can lead to an incorrect structure.
Tips for Drawing Accurate Lewis Structures
To avoid these common mistakes and draw accurate Lewis structures, follow these tips:
- Double-Check Valence Electrons: Always double-check the number of valence electrons for each atom before starting.
- Follow the Octet Rule: Ensure that all atoms (except hydrogen) have a full octet of electrons.
- Use Multiple Bonds When Necessary: If atoms do not have a full octet, form multiple bonds to satisfy the octet rule.
- Draw Clearly and Neatly: A neat and organized diagram will help you avoid mistakes.
- Practice Regularly: The more you practice drawing Lewis structures, the better you will become at it.
HCCH Lewis Structure and Resonance
While the HCCH Lewis structure is straightforward, it's essential to understand that resonance, which involves representing molecules with multiple Lewis structures to depict electron delocalization, is not applicable in this case. The HCCH molecule has a single, well-defined Lewis structure that accurately represents the bonding arrangement. There are no alternative arrangements of electrons that would contribute significantly to the overall structure of the molecule.
Advanced Concepts Related to HCCH Structure
Beyond the basic Lewis structure, several advanced concepts provide a deeper understanding of the HCCH molecule:
- Molecular Orbital Theory: This theory provides a more sophisticated description of bonding in ethyne, considering the interactions of atomic orbitals to form molecular orbitals that span the entire molecule.
- Spectroscopy: Techniques like infrared (IR) spectroscopy and Raman spectroscopy can be used to study the vibrational modes of ethyne, providing experimental evidence for its structure and bonding.
- Computational Chemistry: Computational methods can be used to calculate the electronic structure of ethyne, providing detailed information about its bond lengths, bond angles, and electronic properties.
Conclusion
The HCCH Lewis structure, representing ethyne (acetylene), is a fundamental concept in chemistry that illustrates the principles of covalent bonding, sigma (σ) and pi (π) bonds, and molecular geometry. By understanding the steps involved in constructing the Lewis structure, as well as the related concepts of hybridization and molecular orbital theory, students can gain a deeper appreciation for the structure, properties, and reactivity of this important organic molecule. Its numerous industrial applications further highlight its significance in the broader field of chemistry and materials science. Mastering the HCCH Lewis structure provides a solid foundation for understanding more complex chemical structures and reactions.
Latest Posts
Latest Posts
-
Calories In 1 2 Cup Heavy Cream
Nov 09, 2025
-
Which Two Of The Following Are True About System Software
Nov 09, 2025
-
Identify The Type Of Bonds In This Picture
Nov 09, 2025
-
The Absolute Threshold Is Defined By Psychologists As The
Nov 09, 2025
-
Which Vertebra Lacks Both A Body And Spinous Process
Nov 09, 2025
Related Post
Thank you for visiting our website which covers about H C C H Lewis Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.