How Many Grams Of Oxygen Gas Are Produced When 2.43
arrobajuarez
Nov 06, 2025 · 10 min read
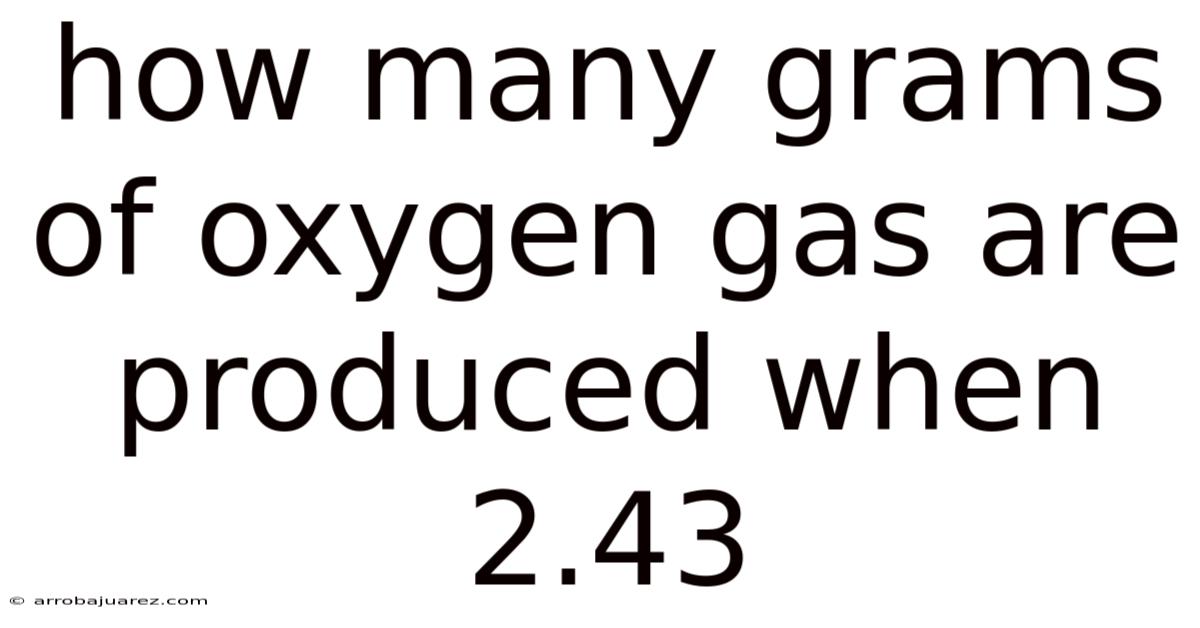
Table of Contents
The Oxygen Unveiled: A Deep Dive into Stoichiometry and Calculations
How many grams of oxygen gas are produced when 2.43 grams of potassium chlorate ($KClO_3$) are heated? This seemingly simple question opens a fascinating door into the world of stoichiometry, chemical reactions, and the fundamental principles that govern them. This article will not only provide the answer but will also guide you through the process step-by-step, ensuring a solid understanding of the concepts involved. We will explore the balanced chemical equation, molar masses, mole ratios, and ultimately, how to convert moles of oxygen to grams. Prepare to delve into the chemistry and unlock the secrets behind this calculation.
Setting the Stage: Decomposition of Potassium Chlorate
Potassium chlorate ($KClO_3$) is a white, crystalline solid that readily decomposes upon heating, producing oxygen gas ($O_2$) and potassium chloride ($KCl$). This reaction is a common method for generating oxygen in laboratory settings and is the basis for our calculation. Understanding the reaction and its balanced chemical equation is the first crucial step.
The Balanced Chemical Equation: Our Roadmap
The balanced chemical equation provides the quantitative relationship between reactants and products. For the decomposition of potassium chlorate, the balanced equation is:
$2KClO_3(s) \longrightarrow 2KCl(s) + 3O_2(g)$
This equation tells us that two moles of potassium chlorate ($KClO_3$) decompose to produce two moles of potassium chloride ($KCl$) and three moles of oxygen gas ($O_2$). This mole ratio is the key to converting between the amount of potassium chlorate we start with and the amount of oxygen gas produced.
Molar Mass: Converting Between Grams and Moles
To perform stoichiometric calculations, we need to work with moles, not grams. The molar mass of a substance is the mass of one mole of that substance, typically expressed in grams per mole (g/mol). We need the molar masses of potassium chlorate ($KClO_3$) and oxygen gas ($O_2$).
-
Molar mass of $KClO_3$:
- Potassium (K): 39.10 g/mol
- Chlorine (Cl): 35.45 g/mol
- Oxygen (O): 16.00 g/mol (and we have 3 of them)
- Molar mass of $KClO_3 = 39.10 + 35.45 + (3 \times 16.00) = 122.55 \text{ g/mol}$
-
Molar mass of $O_2$:
- Oxygen (O): 16.00 g/mol (and we have 2 of them)
- Molar mass of $O_2 = 2 \times 16.00 = 32.00 \text{ g/mol}$
Now that we have the molar masses, we can convert between grams and moles as needed.
The Calculation: A Step-by-Step Guide
Now, let's calculate how many grams of oxygen gas are produced from 2.43 grams of potassium chlorate. We will follow these steps:
- Convert grams of $KClO_3$ to moles of $KClO_3$: Divide the given mass of $KClO_3$ by its molar mass.
- Convert moles of $KClO_3$ to moles of $O_2$: Use the mole ratio from the balanced chemical equation.
- Convert moles of $O_2$ to grams of $O_2$: Multiply the moles of $O_2$ by its molar mass.
Let's do the math!
Step 1: Grams of $KClO_3$ to Moles of $KClO_3$
Moles of $KClO_3 = \frac{\text{Grams of } KClO_3}{\text{Molar mass of } KClO_3} = \frac{2.43 \text{ g}}{122.55 \text{ g/mol}} = 0.0198 \text{ mol}$
Step 2: Moles of $KClO_3$ to Moles of $O_2$
From the balanced equation, $2 \text{ moles } KClO_3 \longrightarrow 3 \text{ moles } O_2$. Therefore, the mole ratio is $\frac{3 \text{ moles } O_2}{2 \text{ moles } KClO_3}$.
Moles of $O_2 = \text{Moles of } KClO_3 \times \text{Mole ratio} = 0.0198 \text{ mol } KClO_3 \times \frac{3 \text{ mol } O_2}{2 \text{ mol } KClO_3} = 0.0297 \text{ mol } O_2$
Step 3: Moles of $O_2$ to Grams of $O_2$
Grams of $O_2 = \text{Moles of } O_2 \times \text{Molar mass of } O_2 = 0.0297 \text{ mol } \times 32.00 \text{ g/mol} = 0.950 \text{ g}$
Therefore, when 2.43 grams of potassium chlorate are heated, approximately 0.950 grams of oxygen gas are produced.
A Visual Summary of the Calculation
Here's a table summarizing the calculation:
| Step | Calculation | Result | Units |
|---|---|---|---|
| 1. $KClO_3$ Grams to Moles | $\frac{2.43 \text{ g } KClO_3}{122.55 \text{ g/mol } KClO_3}$ | 0.0198 | mol $KClO_3$ |
| 2. $KClO_3$ Moles to $O_2$ Moles | $0.0198 \text{ mol } KClO_3 \times \frac{3 \text{ mol } O_2}{2 \text{ mol } KClO_3}$ | 0.0297 | mol $O_2$ |
| 3. $O_2$ Moles to $O_2$ Grams | $0.0297 \text{ mol } O_2 \times 32.00 \text{ g/mol } O_2$ | 0.950 | g $O_2$ |
Factors Affecting Oxygen Yield
While the stoichiometric calculation provides a theoretical yield, several factors can affect the actual amount of oxygen produced in a real-world experiment:
- Purity of the Reactant: Impurities in the potassium chlorate can reduce the amount of $KClO_3$ that actually decomposes, leading to a lower yield of oxygen.
- Completeness of the Reaction: The reaction may not go to completion, meaning that not all of the $KClO_3$ decomposes. This can be due to insufficient heating or other factors.
- Loss of Product: Some oxygen gas may be lost during the experiment due to leaks in the apparatus or incomplete collection.
- Side Reactions: Although the decomposition of potassium chlorate is the primary reaction, other side reactions may occur, consuming some of the $KClO_3$ and reducing the yield of oxygen.
- Catalyst: The presence of a catalyst, such as manganese dioxide ($MnO_2$), can significantly affect the rate of the reaction. While the catalyst doesn't change the theoretical yield (as it's not consumed in the reaction), it allows the reaction to proceed at a lower temperature and potentially more completely in a given time.
Importance of Stoichiometry
Stoichiometry is a fundamental concept in chemistry with broad applications. It allows us to:
- Predict the amount of reactants needed to produce a desired amount of product. This is crucial in industrial chemical processes, where efficiency and cost-effectiveness are paramount.
- Determine the limiting reactant in a reaction. The limiting reactant is the reactant that is completely consumed, determining the maximum amount of product that can be formed.
- Calculate the theoretical yield, actual yield, and percent yield of a reaction. These calculations help us assess the efficiency of a chemical reaction and identify areas for improvement.
- Understand the quantitative relationships between different substances in a chemical reaction. This is essential for interpreting experimental data and developing new chemical processes.
Real-World Applications of Potassium Chlorate Decomposition
The decomposition of potassium chlorate has several practical applications, including:
- Oxygen Generation: As mentioned earlier, it's a convenient method for generating oxygen in laboratory settings.
- Fireworks and Explosives: Potassium chlorate is a strong oxidizing agent and is used in fireworks and explosives to provide oxygen for rapid combustion.
- Matches: It is a component of match heads, providing oxygen to ignite the match.
- Disinfectants and Bleaches: In some applications, it serves as a component in disinfectants and bleaching agents.
- Emergency Oxygen Supply: It can be used in emergency oxygen generators for aircraft or submarines.
Common Mistakes to Avoid
When performing stoichiometric calculations, it's important to avoid common mistakes that can lead to incorrect results:
- Not Balancing the Chemical Equation: An unbalanced equation will lead to incorrect mole ratios and inaccurate calculations.
- Using Incorrect Molar Masses: Double-check the molar masses of all substances involved in the calculation. A small error in molar mass can significantly affect the final result.
- Incorrectly Applying Mole Ratios: Ensure that the mole ratio is set up correctly based on the balanced chemical equation.
- Mixing Up Units: Pay close attention to units and ensure that they are consistent throughout the calculation.
- Rounding Errors: Avoid rounding intermediate values too early in the calculation, as this can lead to significant errors in the final result.
Advanced Considerations: Beyond Ideal Conditions
The calculation we performed assumes ideal conditions. In reality, several factors can complicate the analysis:
- Gas Laws: If the oxygen gas is collected, its volume, pressure, and temperature will affect the amount of oxygen present. The ideal gas law (PV = nRT) can be used to relate these variables.
- Water Vapor Pressure: If the oxygen gas is collected over water, it will be saturated with water vapor. The partial pressure of water vapor must be subtracted from the total pressure to obtain the partial pressure of oxygen.
- Non-Ideal Gas Behavior: At high pressures or low temperatures, oxygen gas may deviate from ideal gas behavior. In these cases, more complex equations of state may be needed.
Expanding Your Knowledge: Related Concepts
To deepen your understanding of stoichiometry and related concepts, consider exploring the following topics:
- Limiting Reactant: Understand how to identify the limiting reactant and its impact on the theoretical yield.
- Percent Yield: Learn how to calculate the percent yield of a reaction and interpret its significance.
- Enthalpy Changes: Explore the enthalpy changes associated with chemical reactions and how they relate to stoichiometry.
- Solution Stoichiometry: Apply stoichiometric principles to reactions in solution, considering molarity and volume.
- Acid-Base Titrations: Use stoichiometry to analyze acid-base titrations and determine the concentration of unknown solutions.
Conclusion: Mastering Stoichiometry
In conclusion, we have successfully determined that approximately 0.950 grams of oxygen gas are produced when 2.43 grams of potassium chlorate are heated. This calculation involved understanding the balanced chemical equation, using molar masses to convert between grams and moles, and applying the mole ratio to relate the amount of $KClO_3$ to the amount of $O_2$. By mastering these concepts, you can confidently tackle a wide range of stoichiometric problems and gain a deeper appreciation for the quantitative nature of chemistry. Stoichiometry is a vital tool for chemists, enabling them to predict, analyze, and optimize chemical reactions in various fields, from research and development to industrial production. Understanding its principles empowers you to unravel the complexities of the chemical world and contribute to scientific advancements.
FAQ: Addressing Common Questions
Here are some frequently asked questions related to the decomposition of potassium chlorate and stoichiometry:
-
Q: What is the role of a catalyst in the decomposition of potassium chlorate?
A: A catalyst, such as manganese dioxide ($MnO_2$), speeds up the reaction without being consumed itself. It lowers the activation energy, allowing the reaction to proceed at a lower temperature. While it increases the rate of the reaction, it doesn't change the amount of oxygen produced from a given amount of potassium chlorate (the theoretical yield).
-
Q: Why is it important to balance the chemical equation before performing stoichiometric calculations?
A: Balancing the chemical equation ensures that the number of atoms of each element is the same on both sides of the equation. This is essential for accurately determining the mole ratios between reactants and products, which are crucial for stoichiometric calculations. An unbalanced equation leads to incorrect mole ratios and inaccurate results.
-
Q: What is the difference between theoretical yield, actual yield, and percent yield?
A: Theoretical yield is the maximum amount of product that can be formed from a given amount of reactant, calculated based on stoichiometry. Actual yield is the amount of product that is actually obtained in a real-world experiment. Percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage. It indicates the efficiency of the reaction.
-
Q: How does the purity of the potassium chlorate affect the amount of oxygen produced?
A: Impurities in the potassium chlorate reduce the amount of actual $KClO_3$ available to decompose. If the 2.43 grams includes impurities, the amount of pure $KClO_3$ is less, leading to a lower yield of oxygen. The calculation we performed assumes that the 2.43 grams is pure $KClO_3$.
-
Q: Can this reaction be reversed?
A: The decomposition of potassium chlorate is generally considered an irreversible reaction under typical laboratory conditions. Reversing the reaction would require extremely high temperatures and pressures, making it impractical.
Latest Posts
Latest Posts
-
What Does The Notation Rr Mean To Geneticists
Nov 06, 2025
-
What Is The S I Unit For Force
Nov 06, 2025
-
What Is The Waste Product Of Photosynthesis
Nov 06, 2025
-
The First Step In Preparing A Flexible Budget Is To
Nov 06, 2025
-
What Is 0 143 As A Whole Number
Nov 06, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Of Oxygen Gas Are Produced When 2.43 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.