How Many Neutrons Are In An Atom Of Mg 25
arrobajuarez
Nov 10, 2025 · 10 min read
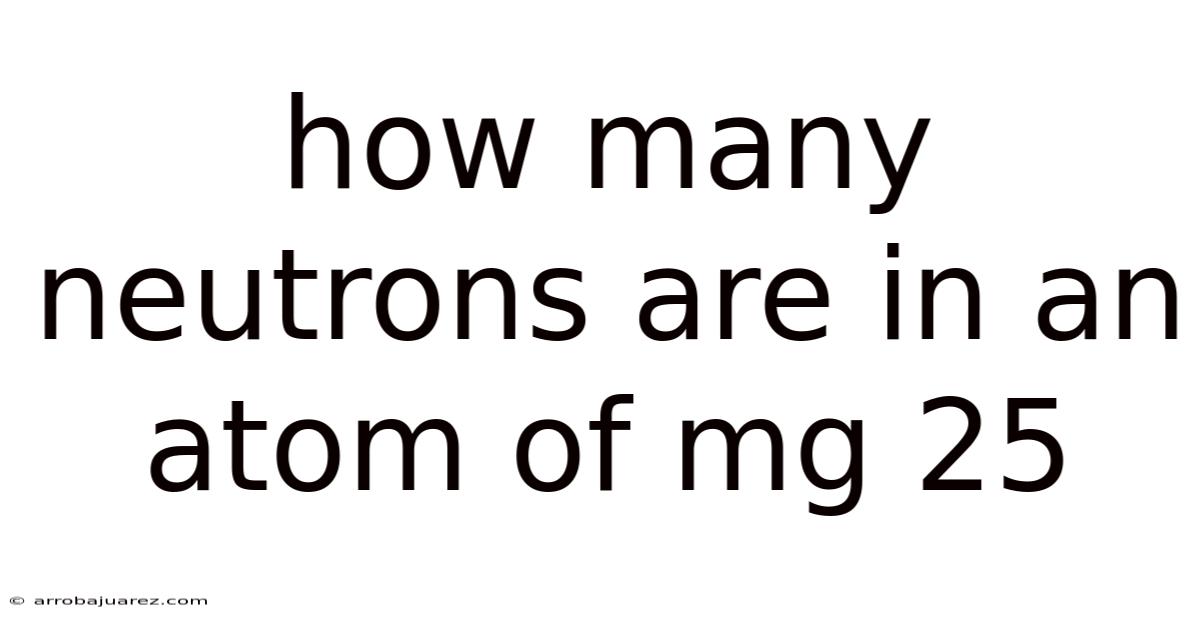
Table of Contents
Understanding the composition of atoms is fundamental to grasping the principles of chemistry and physics. Among the key components of an atom are neutrons, which, along with protons, reside in the nucleus. Determining the number of neutrons in a specific isotope, such as magnesium-25 (Mg-25), involves a straightforward calculation using the atomic mass and atomic number. This article delves into the process of finding the number of neutrons in an atom of Mg-25, providing a comprehensive explanation suitable for readers with varying levels of scientific background.
Introduction to Atomic Structure
Atoms are the basic building blocks of matter. Each atom consists of a nucleus containing protons and neutrons, surrounded by electrons orbiting in various energy levels.
- Protons: Positively charged particles found in the nucleus. The number of protons determines the element's atomic number and, thus, its identity.
- Neutrons: Electrically neutral particles also found in the nucleus. Neutrons contribute to the mass of the atom but do not affect its charge.
- Electrons: Negatively charged particles orbiting the nucleus. The number of electrons in a neutral atom is equal to the number of protons.
The atomic number (Z) represents the number of protons in the nucleus of an atom. For example, all magnesium atoms have an atomic number of 12, meaning they each have 12 protons. The mass number (A), also known as the nucleon number, is the total number of protons and neutrons in an atom's nucleus. It is this mass number that distinguishes different isotopes of the same element.
Isotopes and Atomic Mass
Isotopes are variants of a chemical element which share the same number of protons, but have different numbers of neutrons, and consequently different nucleon numbers. All isotopes of a given element have the same atomic number but different mass numbers. For example, magnesium (Mg) has several isotopes, including magnesium-24 (Mg-24), magnesium-25 (Mg-25), and magnesium-26 (Mg-26). All magnesium isotopes have 12 protons, but they contain 12, 13, and 14 neutrons, respectively.
The atomic mass of an element is the weighted average of the masses of its naturally occurring isotopes. This value is typically expressed in atomic mass units (amu) and can be found on the periodic table. However, when dealing with a specific isotope, such as Mg-25, we refer to its mass number, which is a whole number representing the total count of protons and neutrons.
Determining the Number of Neutrons in Mg-25
To find the number of neutrons in an atom of magnesium-25 (Mg-25), we use the following formula:
Number of Neutrons (N) = Mass Number (A) - Atomic Number (Z)
Where:
- N is the number of neutrons.
- A is the mass number of the isotope.
- Z is the atomic number of the element.
For Mg-25:
- The mass number (A) is 25.
- The atomic number (Z) of magnesium is 12 (since all magnesium atoms have 12 protons).
Plugging these values into the formula:
N = 25 - 12 N = 13
Therefore, an atom of magnesium-25 (Mg-25) contains 13 neutrons.
Step-by-Step Calculation
To illustrate the process further, let's break down the calculation into a step-by-step guide:
- Identify the Isotope:
- In this case, the isotope is magnesium-25 (Mg-25).
- Find the Mass Number (A):
- The mass number is given by the number following the element's name in the isotope notation.
- For Mg-25, the mass number (A) is 25.
- Determine the Atomic Number (Z):
- The atomic number is the number of protons in the nucleus and is unique to each element.
- Magnesium (Mg) has an atomic number of 12. This can be found on the periodic table.
- Apply the Formula:
- Use the formula N = A - Z to calculate the number of neutrons.
- N = 25 - 12
- N = 13
- State the Result:
- An atom of magnesium-25 (Mg-25) contains 13 neutrons.
Importance of Neutron Number
The number of neutrons in an atom's nucleus significantly affects the atom's properties and behavior. While the number of protons defines the element, the number of neutrons influences the stability of the nucleus and the element's isotopic properties.
- Nuclear Stability: The balance between protons and neutrons is crucial for nuclear stability. Nuclei with too few or too many neutrons may be unstable and undergo radioactive decay to achieve a more stable configuration.
- Isotopic Properties: Different isotopes of an element exhibit slightly different physical properties due to the mass difference caused by varying neutron numbers. These differences can be exploited in various applications, such as isotope tracing and nuclear medicine.
- Nuclear Reactions: Neutrons play a critical role in nuclear reactions, such as nuclear fission and nuclear fusion. They can be used to initiate and sustain chain reactions in nuclear reactors and nuclear weapons.
Real-World Applications of Magnesium Isotopes
Magnesium isotopes have various applications across different fields due to their unique properties. Here are a few notable examples:
- Medical Imaging: Some magnesium isotopes are used as contrast agents in medical imaging techniques like MRI (Magnetic Resonance Imaging). They enhance the visibility of certain tissues and organs, aiding in diagnosis.
- Geochemistry: Magnesium isotopes are used to study geological processes, such as the formation of rocks and the cycling of elements in the Earth's crust and mantle. The isotopic composition of magnesium in geological samples can provide insights into their origin and evolution.
- Environmental Science: Magnesium isotopes can be used to trace the sources and transport pathways of pollutants in the environment. By analyzing the isotopic composition of magnesium in water, soil, and air samples, scientists can identify the sources of contamination and assess their impact on ecosystems and human health.
- Materials Science: Magnesium alloys are widely used in various industries due to their lightweight and high strength-to-weight ratio. The isotopic composition of magnesium in these alloys can affect their mechanical and thermal properties, influencing their performance in different applications.
Common Misconceptions
Understanding atomic structure and isotopes can sometimes be confusing due to several common misconceptions. Addressing these misconceptions can help clarify the concepts and prevent errors in calculations and interpretations.
- All Atoms of an Element Are Identical: This is incorrect because isotopes of the same element have different numbers of neutrons and, therefore, different masses. While all atoms of an element have the same number of protons, the varying number of neutrons leads to distinct isotopes.
- Atomic Mass and Mass Number Are the Same: While they are related, they are not the same. The mass number is the total number of protons and neutrons in a specific isotope, while the atomic mass is the weighted average of the masses of all naturally occurring isotopes of an element.
- Electrons Contribute Significantly to Atomic Mass: Electrons have a much smaller mass compared to protons and neutrons. Therefore, their contribution to the overall mass of an atom is negligible. The mass of an atom is primarily determined by the number of protons and neutrons in the nucleus.
- Neutrons Are the Only Particles That Affect Nuclear Stability: While neutrons play a crucial role in nuclear stability, the number of protons and their arrangement in the nucleus also affect stability. The balance between proton-proton repulsion and the strong nuclear force, which holds the nucleus together, determines whether a nucleus is stable or unstable.
Advanced Concepts
For those interested in delving deeper into the topic, here are some advanced concepts related to neutron numbers and atomic structure:
- Nuclear Binding Energy: The energy required to separate a nucleus into its constituent protons and neutrons. The nuclear binding energy is related to the mass defect, which is the difference between the mass of the nucleus and the sum of the masses of its individual protons and neutrons.
- Magic Numbers: Certain numbers of protons or neutrons (2, 8, 20, 28, 50, 82, and 126) result in particularly stable nuclei. These numbers are called magic numbers and are related to the shell structure of the nucleus.
- Neutron Capture: A nuclear reaction in which a nucleus absorbs a neutron, increasing its mass number by one. Neutron capture is an important process in nuclear reactors and in the formation of heavy elements in stars.
- Radioactive Decay: The process by which unstable nuclei transform into more stable nuclei by emitting particles or energy. Radioactive decay can involve the emission of alpha particles (helium nuclei), beta particles (electrons or positrons), or gamma rays (high-energy photons).
Examples of Neutron Number Calculations
To reinforce your understanding, let's look at a few more examples of how to calculate the number of neutrons in different isotopes:
Example 1: Carbon-14 (C-14)
- Mass Number (A) = 14
- Atomic Number (Z) of Carbon = 6
- Number of Neutrons (N) = A - Z = 14 - 6 = 8
- Therefore, Carbon-14 has 8 neutrons.
Example 2: Oxygen-16 (O-16)
- Mass Number (A) = 16
- Atomic Number (Z) of Oxygen = 8
- Number of Neutrons (N) = A - Z = 16 - 8 = 8
- Therefore, Oxygen-16 has 8 neutrons.
Example 3: Uranium-235 (U-235)
- Mass Number (A) = 235
- Atomic Number (Z) of Uranium = 92
- Number of Neutrons (N) = A - Z = 235 - 92 = 143
- Therefore, Uranium-235 has 143 neutrons.
Example 4: Hydrogen-3 (H-3) or Tritium
- Mass Number (A) = 3
- Atomic Number (Z) of Hydrogen = 1
- Number of Neutrons (N) = A - Z = 3 - 1 = 2
- Therefore, Hydrogen-3 (Tritium) has 2 neutrons.
These examples illustrate the straightforward process of determining the number of neutrons in an isotope by subtracting the atomic number from the mass number.
Role of Neutrons in Nuclear Fission
Neutrons play a vital role in nuclear fission, a process in which a heavy nucleus splits into two or more smaller nuclei, releasing a tremendous amount of energy. This process is the basis for nuclear power generation and nuclear weapons.
- Initiation of Fission: Nuclear fission is typically initiated by bombarding a fissile nucleus, such as Uranium-235 (U-235) or Plutonium-239 (Pu-239), with a neutron.
- Neutron Absorption: When a neutron is absorbed by the fissile nucleus, it becomes unstable and splits into two smaller nuclei, along with the release of additional neutrons and energy.
- Chain Reaction: The released neutrons can then go on to strike other fissile nuclei, causing them to undergo fission as well. This creates a self-sustaining chain reaction, in which the number of fission events multiplies rapidly, releasing a large amount of energy in a short period.
- Control of Fission: In nuclear reactors, control rods made of neutron-absorbing materials (such as boron or cadmium) are used to control the rate of the chain reaction. By inserting or withdrawing the control rods, the number of neutrons available to cause fission can be adjusted, allowing the reactor to operate at a desired power level.
The Strong Nuclear Force
The strong nuclear force is one of the four fundamental forces in nature (along with the electromagnetic force, the weak nuclear force, and gravity). It is the force that holds the protons and neutrons together in the nucleus of an atom, overcoming the electrostatic repulsion between the positively charged protons.
- Short-Range Force: The strong nuclear force is a short-range force, meaning it only acts over very short distances (on the order of the size of the nucleus).
- Attractive Force: The strong nuclear force is an attractive force between nucleons (protons and neutrons). This attraction is what holds the nucleus together.
- Charge Independent: The strong nuclear force is approximately charge independent, meaning it acts equally between two protons, two neutrons, or a proton and a neutron.
- Role in Nuclear Stability: The strong nuclear force is essential for nuclear stability. Without it, the electrostatic repulsion between the protons would cause the nucleus to fly apart.
Conclusion
Determining the number of neutrons in an atom, such as magnesium-25 (Mg-25), is a fundamental concept in chemistry and physics. By understanding the atomic number and mass number, one can easily calculate the number of neutrons using the formula N = A - Z. In the case of Mg-25, the number of neutrons is 13. This knowledge is essential for understanding isotopes, nuclear stability, and various applications in medicine, geochemistry, environmental science, and materials science. Moreover, a solid grasp of these concepts helps dispel common misconceptions about atomic structure and lays the foundation for more advanced topics in nuclear physics and chemistry.
Latest Posts
Latest Posts
-
Which Is By Far The Most Common Neuron Type
Nov 10, 2025
-
Consider The Ceo Of A Company That Sells Coffee
Nov 10, 2025
-
The Steel Shaft Is Made From Two Segments
Nov 10, 2025
-
Pension Data For Sterling Properties Include The Following
Nov 10, 2025
-
What Property Of Oil Makes It Float On Water
Nov 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In An Atom Of Mg 25 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.