How Many Oxygen Atoms Are In 110.0 G Of Mg2sio4
arrobajuarez
Oct 29, 2025 · 9 min read
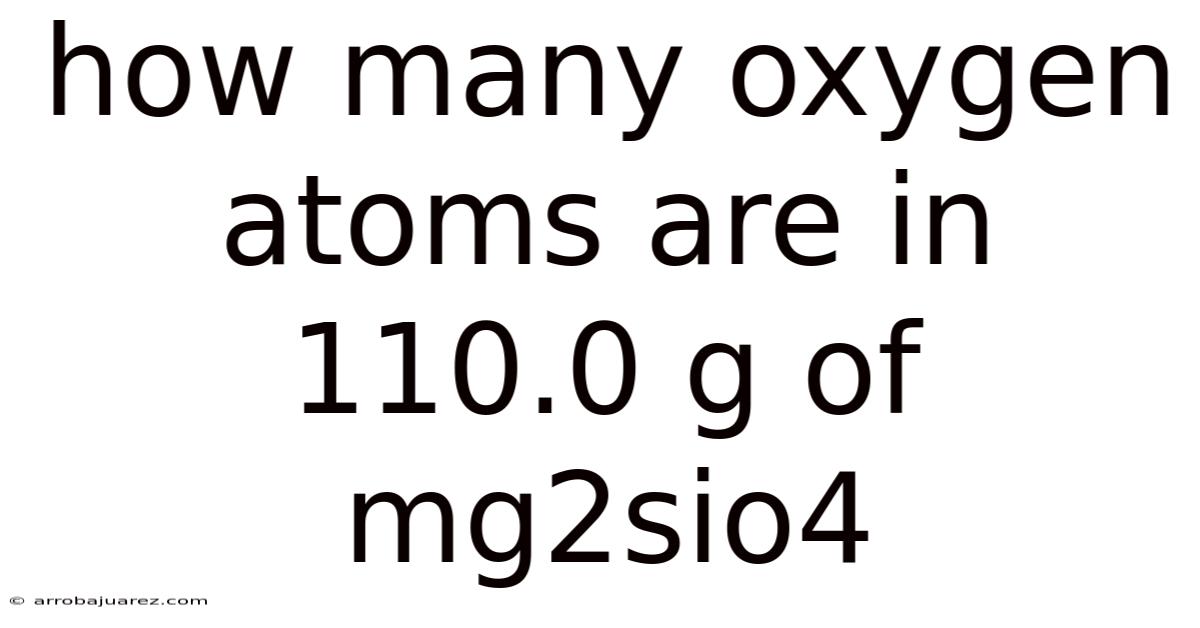
Table of Contents
The quest to understand the microscopic world often leads us to questions like, "How many oxygen atoms are in 110.0 g of Mg₂SiO₄?" This seemingly simple question opens the door to a fascinating journey through stoichiometry, molar mass calculations, and Avogadro's number. By the end of this exploration, you'll not only know the answer but also grasp the underlying principles that govern such calculations.
Understanding the Basics: Magnesium Silicate (Mg₂SiO₄)
Before diving into calculations, let's understand what Mg₂SiO₄, or magnesium silicate, represents. Also known as forsterite, it is a chemical compound composed of magnesium (Mg), silicon (Si), and oxygen (O). The subscript numbers indicate the number of atoms of each element present in one molecule of the compound:
- Mg₂: Two magnesium atoms
- Si: One silicon atom
- O₄: Four oxygen atoms
This understanding is crucial because it forms the foundation for calculating the number of oxygen atoms in a given mass of Mg₂SiO₄.
Key Concepts and Definitions
To accurately determine the number of oxygen atoms, we need to define and understand the following concepts:
- Molar Mass: The mass of one mole of a substance, typically expressed in grams per mole (g/mol).
- Mole: The SI unit of amount of substance, defined as containing exactly 6.02214076 × 10²³ elementary entities (atoms, molecules, ions, etc.). This number is known as Avogadro's number.
- Avogadro's Number (Nᴀ): Approximately 6.022 x 10²³, it represents the number of entities in one mole of a substance.
- Stoichiometry: The quantitative relationship between reactants and products in a chemical reaction. In this context, it refers to the ratio of elements within a compound.
Calculating the Molar Mass of Mg₂SiO₄
The molar mass of Mg₂SiO₄ is the sum of the atomic masses of each element multiplied by the number of atoms of that element in the compound. We'll use the following atomic masses (rounded to two decimal places) from the periodic table:
- Magnesium (Mg): 24.31 g/mol
- Silicon (Si): 28.09 g/mol
- Oxygen (O): 16.00 g/mol
Now, let's calculate the molar mass of Mg₂SiO₄:
Molar Mass of Mg₂SiO₄ = (2 x Atomic Mass of Mg) + (1 x Atomic Mass of Si) + (4 x Atomic Mass of O)
Molar Mass of Mg₂SiO₄ = (2 x 24.31 g/mol) + (1 x 28.09 g/mol) + (4 x 16.00 g/mol)
Molar Mass of Mg₂SiO₄ = 48.62 g/mol + 28.09 g/mol + 64.00 g/mol
Molar Mass of Mg₂SiO₄ = 140.71 g/mol
Therefore, the molar mass of Mg₂SiO₄ is 140.71 g/mol. This value will be crucial in our subsequent calculations.
Step-by-Step Calculation: Oxygen Atoms in 110.0 g of Mg₂SiO₄
Here's a step-by-step guide to calculating the number of oxygen atoms in 110.0 g of Mg₂SiO₄:
Step 1: Convert grams of Mg₂SiO₄ to moles of Mg₂SiO₄
To convert grams to moles, we use the formula:
Moles = Mass / Molar Mass
In this case:
Moles of Mg₂SiO₄ = 110.0 g / 140.71 g/mol
Moles of Mg₂SiO₄ = 0.7817 mol (approximately)
Step 2: Determine the moles of oxygen atoms in the sample
From the chemical formula Mg₂SiO₄, we know that each molecule of magnesium silicate contains four oxygen atoms. Therefore, one mole of Mg₂SiO₄ contains four moles of oxygen atoms.
Moles of O atoms = Moles of Mg₂SiO₄ x 4
Moles of O atoms = 0.7817 mol x 4
Moles of O atoms = 3.1268 mol (approximately)
Step 3: Convert moles of oxygen atoms to the number of oxygen atoms
To convert moles to the number of atoms, we use Avogadro's number (Nᴀ = 6.022 x 10²³ atoms/mol).
Number of O atoms = Moles of O atoms x Avogadro's Number
Number of O atoms = 3.1268 mol x 6.022 x 10²³ atoms/mol
Number of O atoms = 1.883 x 10²⁴ atoms (approximately)
Therefore, there are approximately 1.883 x 10²⁴ oxygen atoms in 110.0 g of Mg₂SiO₄.
Summarizing the Calculation
Let's recap the steps we took to arrive at the answer:
- Calculated the molar mass of Mg₂SiO₄: We determined that the molar mass is 140.71 g/mol.
- Converted grams of Mg₂SiO₄ to moles: We found that 110.0 g of Mg₂SiO₄ is equal to approximately 0.7817 moles.
- Determined moles of oxygen atoms: We established that there are 4 moles of oxygen atoms for every mole of Mg₂SiO₄, resulting in 3.1268 moles of oxygen atoms.
- Converted moles of oxygen atoms to the number of atoms: Using Avogadro's number, we calculated the number of oxygen atoms to be approximately 1.883 x 10²⁴ atoms.
Importance of Stoichiometry in Chemistry
Stoichiometry is a fundamental concept in chemistry that allows us to understand the quantitative relationships between elements and compounds. It's not just about performing calculations; it's about understanding the composition of matter at a molecular level. Here's why it's important:
- Predicting Reaction Outcomes: Stoichiometry enables chemists to predict the amount of product formed in a chemical reaction based on the amount of reactants used.
- Determining Empirical Formulas: By analyzing the mass composition of a compound, stoichiometry can be used to determine the empirical formula, which represents the simplest whole-number ratio of atoms in the compound.
- Balancing Chemical Equations: Stoichiometry is essential for balancing chemical equations, ensuring that the number of atoms of each element is conserved during a chemical reaction.
- Quantitative Analysis: In analytical chemistry, stoichiometry is used to quantify the amount of a particular substance in a sample.
Real-World Applications
The principles of stoichiometry extend far beyond the classroom and are applied in numerous real-world scenarios:
- Pharmaceutical Industry: Stoichiometry is crucial for ensuring the correct dosages of medications.
- Manufacturing: Industries rely on stoichiometry to optimize production processes and minimize waste.
- Environmental Science: Stoichiometry is used to analyze pollutants and understand chemical reactions in the environment.
- Materials Science: Understanding the composition of materials at the atomic level is critical for designing and developing new materials with specific properties.
- Agriculture: Stoichiometry helps farmers determine the correct amount of fertilizers to use for optimal crop growth.
Common Mistakes and How to Avoid Them
While the calculations involved in stoichiometry can be straightforward, there are some common mistakes that students and even experienced chemists can make. Here are some pitfalls to avoid:
- Incorrect Molar Masses: Using incorrect molar masses is a common source of error. Always double-check the atomic masses from the periodic table and ensure you're using the correct values.
- Misinterpreting Chemical Formulas: Failing to correctly interpret chemical formulas can lead to errors in determining the ratio of atoms in a compound. Pay close attention to subscripts and parentheses.
- Unit Conversions: Always ensure that you're using consistent units throughout your calculations. Convert all masses to grams and use molar masses in g/mol.
- Rounding Errors: Rounding intermediate values too early can introduce significant errors in the final result. It's best to carry out calculations with as many significant figures as possible and round only at the end.
- Forgetting Avogadro's Number: When converting moles to the number of atoms or molecules, remember to use Avogadro's number.
Advanced Stoichiometry: Beyond Simple Calculations
While this article focuses on a relatively simple stoichiometric calculation, the principles can be extended to more complex scenarios. Some advanced topics include:
- Limiting Reactants: In chemical reactions, the limiting reactant is the reactant that is completely consumed first, thereby limiting the amount of product that can be formed.
- Percent Yield: The percent yield is the ratio of the actual yield (the amount of product obtained in a reaction) to the theoretical yield (the amount of product predicted by stoichiometry), expressed as a percentage.
- Solution Stoichiometry: This involves performing stoichiometric calculations for reactions that occur in solutions, taking into account the concentrations of the reactants.
- Gas Stoichiometry: This deals with reactions involving gases, using the ideal gas law to relate the volume, pressure, and temperature of the gases to the amount of reactants and products.
The Importance of Accurate Calculations
In many scientific and industrial applications, accurate stoichiometric calculations are essential for safety, efficiency, and cost-effectiveness. For example:
- Chemical Synthesis: In the synthesis of new compounds, accurate stoichiometry is critical for ensuring that the desired product is obtained in the highest possible yield.
- Environmental Monitoring: Accurate measurements of pollutants in the environment require precise stoichiometric calculations.
- Food Production: In the food industry, stoichiometry is used to ensure the correct proportions of ingredients in food products.
- Energy Production: In power plants, stoichiometry is used to optimize the combustion of fuels and minimize emissions.
The Role of Technology
Modern technology plays a significant role in facilitating stoichiometric calculations. There are numerous software programs and online calculators that can perform complex calculations quickly and accurately. These tools can be particularly useful in industrial settings where large numbers of calculations are required. However, it's important to remember that these tools are only as good as the data entered into them, and a thorough understanding of the underlying principles of stoichiometry is still essential.
Magnesium Silicate in Nature and Industry
Magnesium silicate, or forsterite, is not just a chemical compound for calculations; it's a naturally occurring mineral with various applications. It is a major component of the Earth's mantle and is also found in meteorites. Here are some interesting facts about magnesium silicate:
- Occurrence: Forsterite is found in igneous and metamorphic rocks and is often associated with other minerals such as olivine and peridot.
- Industrial Uses: Magnesium silicate is used in the production of refractory materials, which are heat-resistant materials used in high-temperature applications such as furnaces and kilns. It is also used as a catalyst and as an absorbent.
- Gemstones: Some varieties of forsterite are used as gemstones, known as peridot.
- Health and Safety: Magnesium silicate is generally considered to be safe, but inhalation of dust may cause respiratory irritation.
Conclusion: The Power of Quantitative Analysis
Calculating the number of oxygen atoms in 110.0 g of Mg₂SiO₄ is more than just an exercise in stoichiometry. It's a demonstration of the power of quantitative analysis in chemistry. By understanding the relationships between elements and compounds at the atomic level, we can gain valuable insights into the properties and behavior of matter. These insights have far-reaching implications for science, industry, and our understanding of the world around us. Mastering these calculations unlocks a deeper appreciation for the precision and elegance of chemistry, and it provides a foundation for further exploration of the molecular world. From predicting reaction outcomes to designing new materials, the principles of stoichiometry are essential tools for chemists and scientists in a wide range of fields. The journey from grams to atoms is a testament to the power of quantitative thinking and the beauty of chemistry.
Latest Posts
Related Post
Thank you for visiting our website which covers about How Many Oxygen Atoms Are In 110.0 G Of Mg2sio4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.