In The Structure Of 4-isopropyl-2 4 5-trimethylheptane
arrobajuarez
Nov 11, 2025 · 9 min read
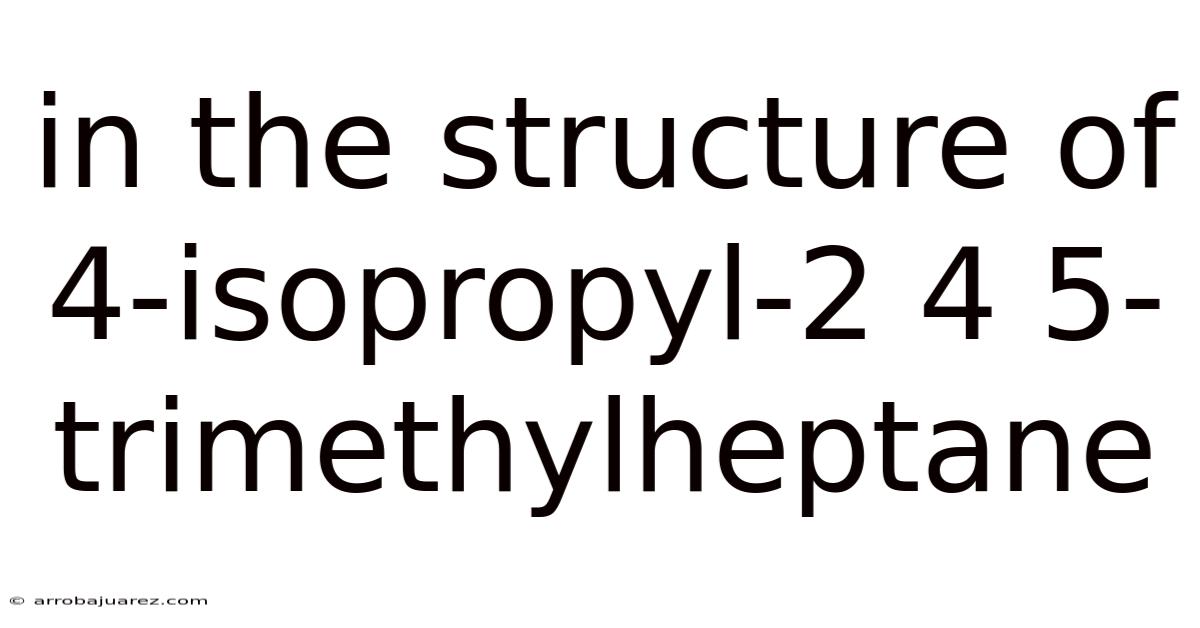
Table of Contents
Diving into the molecular architecture of 4-isopropyl-2,4,5-trimethylheptane unveils a fascinating world of organic chemistry, where the arrangement of atoms dictates the properties and behavior of this complex hydrocarbon. Understanding its structure is essential for predicting its chemical reactivity, physical characteristics, and potential applications in various fields.
Decoding the Name: 4-isopropyl-2,4,5-trimethylheptane
The name itself provides a roadmap to the molecule's structure. Let's break it down piece by piece:
- Heptane: This indicates the parent chain is a straight chain of seven carbon atoms. This forms the backbone of the molecule.
- 2,4,5-trimethyl: This tells us that there are three methyl groups (CH3) attached to the heptane chain at positions 2, 4, and 5. Numbering the carbon atoms in the heptane chain allows us to pinpoint the exact location of these substituents.
- 4-isopropyl: This indicates that an isopropyl group (a branched alkyl group with the structure CH(CH3)2) is attached to the heptane chain at position 4.
By combining these elements, we can visualize the complete structure of 4-isopropyl-2,4,5-trimethylheptane.
Building the Structure Step-by-Step
Let's construct the molecule from the ground up:
-
Draw the Heptane Backbone: Start by drawing a straight chain of seven carbon atoms. This is the fundamental framework of the molecule. Number the carbons 1 through 7 for clarity.
1-2-3-4-5-6-7 -
Add the Methyl Groups: At carbon positions 2, 4, and 5, attach a methyl group (CH3) to each carbon.
CH3 | 1-2-3-4-5-6-7 | | | CH3 CH3 CH3 -
Incorporate the Isopropyl Group: At carbon position 4, attach an isopropyl group (CH(CH3)2). This group consists of a central carbon bonded to a hydrogen atom and two methyl groups.
CH3 | CH3-CH | 1-2-3-4-5-6-7 | | | CH3 CH3 CH3 -
Complete the Hydrogen Atoms: Finally, add hydrogen atoms to each carbon atom to ensure that each carbon has four bonds (octet rule). This completes the structure of 4-isopropyl-2,4,5-trimethylheptane.
Visualizing the 3D Structure
While we can draw the molecule on paper, it's important to remember that molecules exist in three-dimensional space. The arrangement of atoms in 3D significantly impacts the molecule's properties.
- Tetrahedral Geometry: Each carbon atom with four single bonds adopts a tetrahedral geometry. This means the four groups bonded to the carbon atom are arranged in a tetrahedron, with the carbon at the center and the four groups at the corners.
- Bond Angles: The bond angles around each tetrahedral carbon are approximately 109.5 degrees. This arrangement minimizes repulsion between the electron pairs in the bonds.
- Conformations: The molecule can adopt different conformations due to the rotation of single bonds. These conformations represent different spatial arrangements of the atoms. Some conformations are more stable than others due to steric interactions (repulsion between bulky groups). The most stable conformation will generally minimize these steric interactions.
Key Structural Features
The structure of 4-isopropyl-2,4,5-trimethylheptane has several key features:
- High Branching: The molecule is highly branched due to the presence of the isopropyl and methyl groups. This branching significantly influences its physical properties.
- Steric Hindrance: The multiple substituents on the heptane chain create significant steric hindrance. This means that the bulky groups impede the approach of other molecules or atoms, affecting its reactivity.
- Chirality: The carbon atom at position 4 is a chiral center because it is bonded to four different groups: a methyl group, an isopropyl group, and two different alkyl chains. This chirality means that the molecule can exist as two non-superimposable mirror images, called enantiomers.
Physical Properties Influenced by Structure
The unique structure of 4-isopropyl-2,4,5-trimethylheptane directly influences its physical properties:
- Boiling Point: The high degree of branching reduces the boiling point compared to a straight-chain alkane with the same number of carbon atoms. Branching decreases the surface area of the molecule, reducing the intermolecular forces (van der Waals forces) between molecules. Lower intermolecular forces translate to a lower boiling point.
- Melting Point: The branching also affects the melting point. The irregular shape of the molecule makes it difficult to pack efficiently into a crystal lattice. This results in weaker intermolecular forces and a lower melting point.
- Density: The branching generally leads to a lower density compared to a straight-chain alkane.
- Viscosity: The molecule's viscosity is influenced by its shape and intermolecular forces. Highly branched alkanes tend to have lower viscosities.
Chemical Reactivity and Structure
The structure also plays a crucial role in determining the chemical reactivity of 4-isopropyl-2,4,5-trimethylheptane:
- Inertness: Alkanes, in general, are relatively unreactive due to the strong C-C and C-H bonds and the lack of a significant dipole moment.
- Combustion: Alkanes readily undergo combustion, reacting with oxygen to produce carbon dioxide and water, releasing a significant amount of energy. The branching can affect the efficiency of combustion.
- Free-Radical Reactions: Alkanes can participate in free-radical reactions, such as halogenation. The stability of the resulting free radical intermediate is influenced by the degree of substitution at the carbon atom. Tertiary carbons (carbons bonded to three other carbon atoms) form more stable free radicals than secondary or primary carbons.
- Steric Effects: The steric hindrance around the molecule can hinder reactions that require the approach of a bulky reagent.
Applications and Relevance
While 4-isopropyl-2,4,5-trimethylheptane may not have widespread specific applications due to its complexity and the potential cost of synthesis, understanding its structure and properties is valuable for several reasons:
- Petroleum Chemistry: Complex branched alkanes like this are often found in crude oil. Understanding their properties is crucial for refining and processing petroleum.
- Model Compound: It can serve as a model compound for studying the relationship between molecular structure and physical properties in more complex hydrocarbon systems.
- Organic Synthesis: The principles involved in understanding its structure are fundamental to organic synthesis, where chemists design and build complex molecules with specific properties.
- Pharmaceutical Chemistry: The principles of steric hindrance, chirality, and conformational analysis are crucial in drug design, where the shape and size of a molecule determine its interaction with biological targets.
Isomers and Structural Variations
It's important to note that there are many possible isomers of 4-isopropyl-2,4,5-trimethylheptane. Isomers are molecules with the same molecular formula (same number and types of atoms) but different structural arrangements. These structural variations can lead to significantly different properties.
- Constitutional Isomers: These isomers have different connectivity of atoms. For example, we could move one of the methyl groups to a different position on the heptane chain, resulting in a different constitutional isomer.
- Stereoisomers: These isomers have the same connectivity but differ in the spatial arrangement of atoms. Enantiomers are a type of stereoisomer.
Advanced Spectroscopic Techniques for Structure Elucidation
If presented with an unknown sample of 4-isopropyl-2,4,5-trimethylheptane, how would chemists confirm its structure? Several advanced spectroscopic techniques are used to elucidate the structure of organic molecules:
- Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy provides detailed information about the carbon and hydrogen atoms in the molecule. Different carbon and hydrogen atoms in different chemical environments will give rise to distinct signals in the NMR spectrum. The number of signals, their positions (chemical shifts), and their splitting patterns (multiplicity) provide valuable information about the connectivity and environment of each atom.
- Infrared (IR) Spectroscopy: IR spectroscopy provides information about the functional groups present in the molecule. While alkanes lack distinctive functional groups, IR spectroscopy can confirm the presence of C-H bonds and the absence of other functional groups.
- Mass Spectrometry (MS): Mass spectrometry provides information about the molecular weight of the molecule and its fragmentation pattern. The fragmentation pattern can provide clues about the structure of the molecule.
- X-ray Crystallography: If a single crystal of the compound can be obtained, X-ray crystallography can provide a precise three-dimensional structure of the molecule.
The Importance of Conformational Analysis
As mentioned earlier, 4-isopropyl-2,4,5-trimethylheptane can exist in different conformations due to rotation around the single bonds. Conformational analysis is the study of these different conformations and their relative energies. Understanding the conformational preferences of a molecule is crucial for predicting its reactivity and interactions with other molecules.
- Newman Projections: Newman projections are a useful tool for visualizing the different conformations of a molecule. They show the molecule looking down a particular carbon-carbon bond. The substituents on the front and back carbons are drawn, and the angle between them is called the dihedral angle.
- Steric Strain: Different conformations have different energies due to steric strain. Steric strain arises from the repulsion between bulky groups that are close to each other in space.
- Torsional Strain: Torsional strain arises from the repulsion between electron pairs in bonds that are eclipsed (dihedral angle of 0 degrees).
- Gauche Interactions: A gauche interaction occurs when two bulky groups are separated by a dihedral angle of 60 degrees. Gauche interactions are less stable than anti interactions (dihedral angle of 180 degrees).
For 4-isopropyl-2,4,5-trimethylheptane, the conformational analysis would involve considering the various rotations around the C-C bonds and identifying the conformations that minimize steric and torsional strain. The most stable conformation would likely be the one where the bulky isopropyl group and methyl groups are as far apart as possible.
Computational Chemistry and Molecular Modeling
Modern computational chemistry tools can be used to model the structure and properties of 4-isopropyl-2,4,5-trimethylheptane. These tools can provide valuable insights into its conformation, energy, and reactivity.
- Molecular Mechanics: Molecular mechanics methods use classical mechanics to calculate the energy of a molecule based on its geometry. These methods are computationally fast and can be used to study large molecules.
- Quantum Mechanics: Quantum mechanics methods use the principles of quantum mechanics to calculate the electronic structure of a molecule. These methods are more computationally demanding but provide more accurate results.
- Density Functional Theory (DFT): DFT is a widely used quantum mechanical method that provides a good balance between accuracy and computational cost.
By using these computational tools, chemists can gain a deeper understanding of the structure and properties of 4-isopropyl-2,4,5-trimethylheptane and other complex organic molecules.
Conclusion
The structure of 4-isopropyl-2,4,5-trimethylheptane, while complex, can be systematically understood by breaking down its name and considering the principles of organic chemistry. Its highly branched structure influences its physical properties, chemical reactivity, and conformational preferences. Understanding the structure of this molecule provides a valuable foundation for understanding the behavior of more complex organic molecules and for designing new molecules with specific properties. Through the application of spectroscopic techniques, conformational analysis, and computational chemistry, chemists can gain a comprehensive understanding of this fascinating molecule and its role in the broader world of organic chemistry.
Latest Posts
Latest Posts
-
Which Statement About Groupthink Is Correct
Nov 11, 2025
-
Compute The Average Manufacturing Cost Per Drum Set
Nov 11, 2025
-
Internal Recruiting Is Helpful If A Company Wants To
Nov 11, 2025
-
Which Is Not A Function Of Bone
Nov 11, 2025
-
Calculate The Rotational Inertia Of A Meter Stick
Nov 11, 2025
Related Post
Thank you for visiting our website which covers about In The Structure Of 4-isopropyl-2 4 5-trimethylheptane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.