Research On ________ Subjects Must Always Involve ________.
arrobajuarez
Nov 09, 2025 · 11 min read
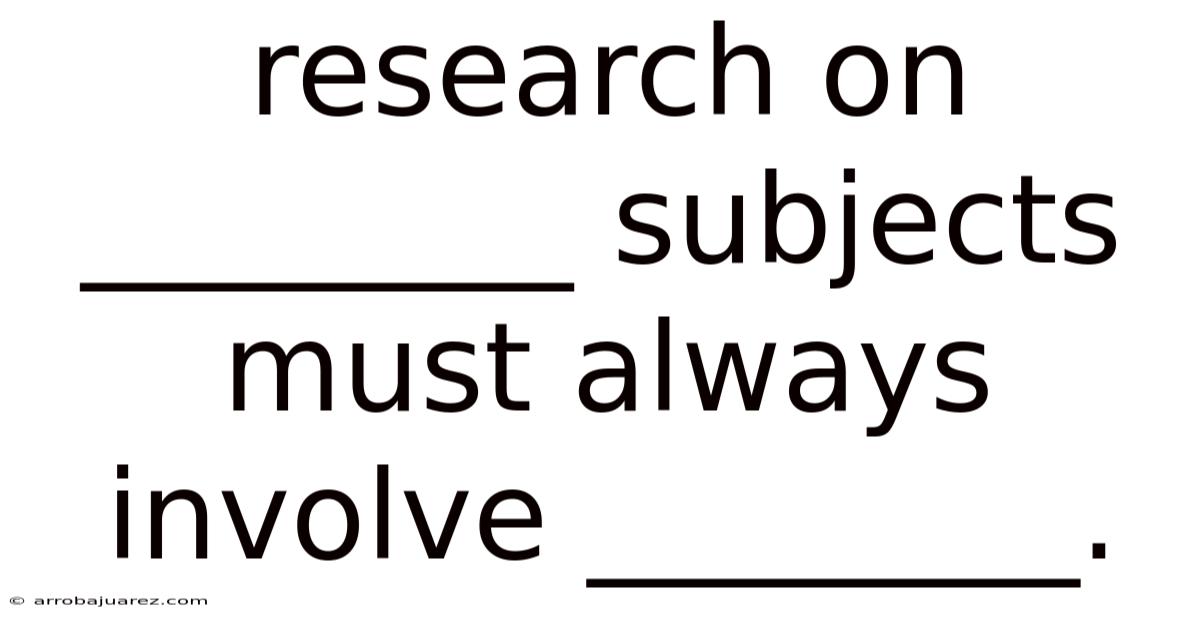
Table of Contents
Research on human subjects must always involve informed consent. This principle is not merely a procedural formality; it is the cornerstone of ethical research, ensuring respect for individuals and safeguarding their autonomy. Informed consent acknowledges that every person has the right to decide whether or not to participate in a study, based on a clear understanding of what the research entails. Without informed consent, research risks becoming exploitative, disrespectful, and potentially harmful.
Understanding Informed Consent
Informed consent is more than just a signature on a form. It’s a comprehensive process designed to protect the rights and well-being of research participants. It encompasses several key elements, each contributing to ensuring that participation is truly voluntary and based on adequate information.
- Voluntariness: Participation must be entirely voluntary and free from coercion or undue influence. Individuals should feel empowered to decline participation or withdraw from the study at any time without penalty.
- Disclosure: Researchers must provide a clear and comprehensive explanation of the research, including its purpose, procedures, potential risks and benefits, alternatives to participation, and the confidentiality measures in place.
- Comprehension: Information must be presented in a language and format that the participant can understand. Researchers have a responsibility to ensure that participants truly grasp the information provided, and should be prepared to answer questions and address concerns.
- Competence: Participants must be competent to make an informed decision. This means they must have the capacity to understand the information presented and appreciate the consequences of their decision. If a participant lacks competence (e.g., due to age, cognitive impairment), a legally authorized representative must provide consent on their behalf.
The Historical Context: Why Informed Consent Matters
The necessity of informed consent emerged from a dark history of unethical research practices. Throughout the 20th century, numerous studies were conducted that violated basic human rights, highlighting the urgent need for ethical guidelines and regulations.
- The Nazi Medical Experiments: During World War II, Nazi doctors conducted horrific experiments on concentration camp prisoners, subjecting them to torture and abuse under the guise of scientific research. These experiments exposed the dangers of research conducted without consent or ethical oversight.
- The Tuskegee Syphilis Study: From 1932 to 1972, the U.S. Public Health Service conducted a study on hundreds of African American men with syphilis, without informing them of their diagnosis or providing them with effective treatment, even after penicillin became available. This study is a stark reminder of the devastating consequences of exploiting vulnerable populations in research.
- The Willowbrook State School Study: In the 1960s and 70s, children with intellectual disabilities at the Willowbrook State School in New York were deliberately infected with hepatitis in order to study the course of the disease. This study raised serious ethical concerns about the vulnerability of institutionalized populations and the potential for exploitation in research.
These historical atrocities led to the development of international ethical guidelines, such as the Nuremberg Code and the Belmont Report, which emphasize the importance of informed consent, respect for persons, beneficence, and justice in research.
The Elements of an Informed Consent Form
The informed consent form serves as a written record of the consent process, documenting that the participant has been provided with the necessary information and has agreed to participate in the study. While the specific content of the form may vary depending on the nature of the research, it typically includes the following elements:
- Study Title and Purpose: A clear and concise explanation of the research question and the overall goals of the study.
- Procedures: A detailed description of the research procedures, including what the participant will be asked to do, how long the study will last, and any potential risks or discomforts.
- Risks and Benefits: A comprehensive assessment of the potential risks and benefits of participation, both to the individual participant and to society as a whole.
- Confidentiality: An explanation of how the participant's data will be protected and kept confidential, including who will have access to the data and how it will be stored and used.
- Voluntary Participation and Right to Withdraw: A clear statement that participation is voluntary and that the participant has the right to withdraw from the study at any time without penalty.
- Contact Information: Contact information for the researcher and the Institutional Review Board (IRB) or ethics committee, so that participants can ask questions or raise concerns.
- Statement of Consent: A clear statement that the participant understands the information provided and agrees to participate in the study.
- Signature and Date: A space for the participant to sign and date the form, indicating their consent.
Challenges to Obtaining Informed Consent
While informed consent is a fundamental ethical principle, there are situations in which obtaining it can be challenging. These challenges may arise due to various factors, such as:
- Vulnerable Populations: Certain populations, such as children, individuals with cognitive impairments, prisoners, and individuals with limited English proficiency, may be particularly vulnerable to coercion or undue influence and may have difficulty understanding the information presented.
- Emergency Situations: In emergency situations, it may not be possible to obtain informed consent before providing medical treatment. In such cases, researchers may need to rely on alternative mechanisms, such as surrogate consent or emergency waivers.
- Cultural Differences: Cultural norms and values can influence individuals' understanding of informed consent and their willingness to participate in research. Researchers need to be sensitive to these cultural differences and adapt their consent procedures accordingly.
- Online Research: Conducting research online presents unique challenges to obtaining informed consent, as it can be difficult to verify the identity of participants and ensure that they are fully informed about the risks and benefits of participation.
Strategies for Improving Informed Consent
To address these challenges, researchers can employ various strategies to improve the informed consent process.
- Using Plain Language: Presenting information in clear, concise, and easy-to-understand language, avoiding jargon and technical terms.
- Employing Visual Aids: Utilizing visual aids, such as diagrams, charts, and videos, to enhance comprehension.
- Providing Oral Explanations: Supplementing written consent forms with oral explanations and opportunities for participants to ask questions.
- Assessing Comprehension: Implementing methods to assess participants' understanding of the information provided, such as asking them to summarize the key points or answer comprehension questions.
- Utilizing Community Engagement: Engaging with community representatives and stakeholders to ensure that research is culturally sensitive and responsive to community needs.
- Developing Innovative Consent Models: Exploring innovative consent models, such as dynamic consent, which allows participants to have ongoing control over their data and participation.
The Role of Institutional Review Boards (IRBs)
Institutional Review Boards (IRBs), also known as ethics committees, play a crucial role in protecting the rights and welfare of human research participants. IRBs are responsible for reviewing and approving research protocols to ensure that they comply with ethical guidelines and regulations.
- Reviewing Research Protocols: IRBs review research protocols to assess the potential risks and benefits of the study, the adequacy of the informed consent process, and the protection of vulnerable populations.
- Monitoring Ongoing Research: IRBs monitor ongoing research to ensure that researchers are adhering to the approved protocol and that any adverse events are promptly reported and addressed.
- Providing Guidance and Education: IRBs provide guidance and education to researchers on ethical research practices and regulatory requirements.
- Ensuring Compliance: IRBs have the authority to approve, disapprove, or require modifications to research protocols to ensure compliance with ethical standards.
Informed Consent in Specific Research Contexts
The principles of informed consent apply to all types of research involving human subjects, but the specific implementation may vary depending on the context.
- Clinical Trials: In clinical trials, participants must be informed about the investigational nature of the treatment, the potential risks and benefits, and the alternatives to participation.
- Social and Behavioral Research: In social and behavioral research, participants must be informed about the purpose of the study, the procedures involved, and the potential risks to privacy and confidentiality.
- Genetic Research: In genetic research, participants must be informed about the potential implications of genetic testing, including the possibility of discovering unexpected findings about their health or ancestry.
- Research with Children: When conducting research with children, researchers must obtain assent from the child (if they are capable of understanding) and consent from their parents or legal guardians.
- Research with Individuals with Cognitive Impairments: When conducting research with individuals with cognitive impairments, researchers must assess their capacity to understand the information presented and obtain consent from a legally authorized representative if necessary.
The Future of Informed Consent
As research continues to evolve and new technologies emerge, the concept of informed consent must adapt to meet the challenges of the 21st century.
- Digital Consent: Developing secure and user-friendly digital consent platforms that allow participants to provide consent electronically and manage their data preferences.
- Dynamic Consent: Implementing dynamic consent models that allow participants to have ongoing control over their data and participation, enabling them to update their preferences and withdraw their consent at any time.
- Broad Consent: Exploring the use of broad consent for future research, allowing participants to consent to the use of their data for a wide range of unspecified research purposes, while still maintaining appropriate safeguards for privacy and confidentiality.
- Artificial Intelligence (AI) and Informed Consent: Addressing the ethical implications of using AI in research, including ensuring that participants are informed about how AI is being used to analyze their data and make decisions about their care.
- Global Perspectives on Informed Consent: Promoting international collaboration to harmonize ethical standards for research involving human subjects, ensuring that all participants are treated with respect and dignity, regardless of their location.
Legal and Ethical Ramifications of Ignoring Informed Consent
Failure to obtain informed consent can have serious legal and ethical consequences for researchers and institutions.
- Legal Liability: Researchers who conduct research without informed consent may be subject to lawsuits for negligence, battery, or other torts. Institutions may also be held liable for the actions of their researchers.
- Regulatory Sanctions: Research that violates ethical guidelines and regulations may be subject to sanctions by regulatory agencies, such as the Food and Drug Administration (FDA) or the Office for Human Research Protections (OHRP). These sanctions may include fines, suspension of research funding, or debarment from conducting research.
- Reputational Damage: Conducting unethical research can damage the reputation of researchers and institutions, eroding public trust in science and undermining the credibility of research findings.
- Ethical Condemnation: Research that violates ethical principles may be condemned by professional organizations, ethics committees, and the broader scientific community.
Practical Examples of Informed Consent in Action
To illustrate the importance of informed consent in practice, consider the following examples:
- A researcher is conducting a study to evaluate the effectiveness of a new medication for treating depression. Before enrolling participants in the study, the researcher provides them with a detailed informed consent form that explains the purpose of the study, the procedures involved, the potential risks and benefits of the medication, and the alternatives to participation. The researcher also takes the time to answer participants' questions and address their concerns.
- A sociologist is conducting interviews with members of a marginalized community to understand their experiences with discrimination. Before conducting the interviews, the sociologist obtains informed consent from each participant, explaining the purpose of the study, how their data will be used, and the measures that will be taken to protect their privacy and confidentiality.
- A geneticist is collecting DNA samples from individuals to study the genetic basis of a particular disease. Before collecting the samples, the geneticist obtains informed consent from each participant, explaining the purpose of the study, the potential risks and benefits of genetic testing, and the options for receiving information about their genetic results.
In each of these examples, informed consent is essential to ensuring that participants are treated with respect and dignity and that their rights and welfare are protected.
Conclusion
Informed consent is not just a regulatory requirement; it is a fundamental ethical imperative in research involving human subjects. It upholds the principles of autonomy, respect, and beneficence, ensuring that individuals have the right to make informed decisions about their participation in research. While challenges to obtaining informed consent exist, ongoing efforts to improve the process, coupled with rigorous oversight by IRBs, are essential to safeguarding the rights and well-being of research participants. As research continues to advance, the principles of informed consent must remain at the forefront, guiding ethical practices and fostering trust between researchers and the public. Prioritizing informed consent is not only ethically sound, but also crucial for promoting credible and impactful research that benefits society as a whole. By embracing informed consent as a core value, we can ensure that research serves humanity while upholding the dignity and rights of every individual.
Latest Posts
Latest Posts
-
Which Of The Following Is A Correct Statement
Nov 09, 2025
-
Use The Figure At The Right
Nov 09, 2025
-
Which Of The Following Is Vector
Nov 09, 2025
-
Pens And Corrals In Vertex Form
Nov 09, 2025
-
Which Countries Would Benefit Most From Fuel Made From Seawater
Nov 09, 2025
Related Post
Thank you for visiting our website which covers about Research On ________ Subjects Must Always Involve ________. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.