When 2.50 G Of Copper Reacts With Oxygen
arrobajuarez
Nov 06, 2025 · 10 min read
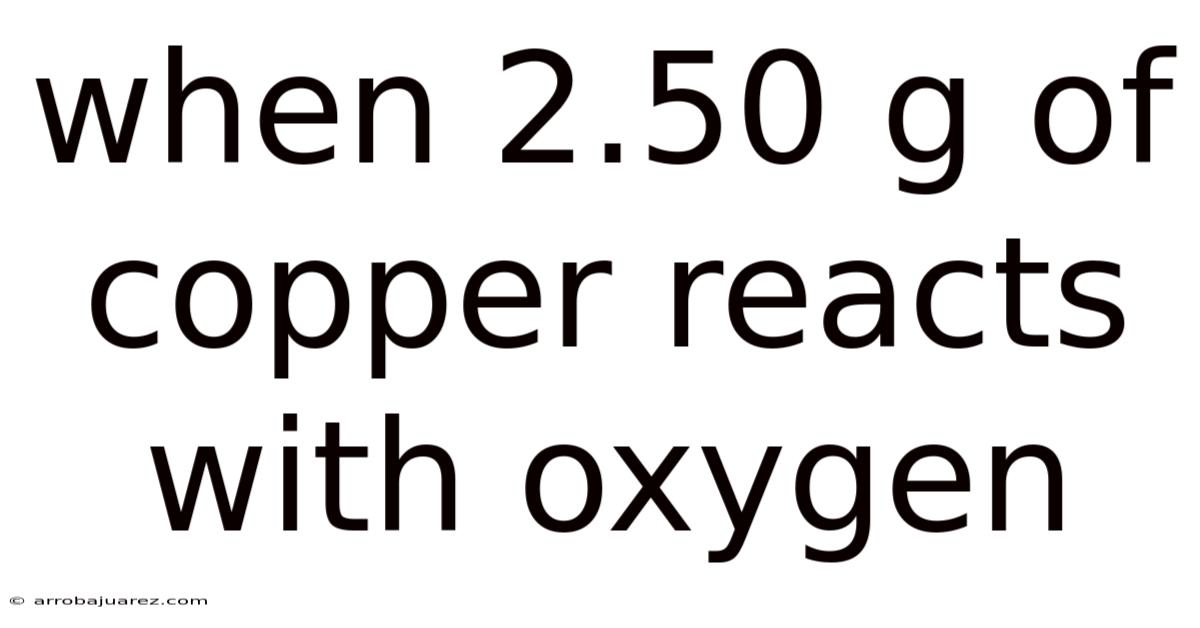
Table of Contents
The interaction between copper and oxygen, a fundamental chemical reaction, is a process that reveals the intricacies of oxidation, stoichiometry, and the properties of metallic compounds. When 2.50 grams of copper reacts with oxygen, it undergoes a transformation that results in the formation of copper oxide, a compound with significant implications in various industrial applications and everyday phenomena.
Understanding the Reaction
At its core, the reaction between copper (Cu) and oxygen (O₂) is an oxidation-reduction (redox) process. Copper atoms lose electrons (oxidation) to oxygen atoms, which gain electrons (reduction), resulting in the formation of copper oxide. The type of copper oxide formed depends on the reaction conditions, particularly the availability of oxygen and temperature. Two primary forms of copper oxide can be produced:
-
Copper(I) Oxide (Cu₂O): Also known as cuprous oxide, this compound is typically red in color and is formed under conditions where oxygen is limited.
-
Copper(II) Oxide (CuO): Also known as cupric oxide, this compound is black in color and is formed when copper reacts with an excess of oxygen.
The balanced chemical equations for these reactions are:
- For Copper(I) Oxide:
4Cu(s) + O₂(g) → 2Cu₂O(s) - For Copper(II) Oxide:
2Cu(s) + O₂(g) → 2CuO(s)
Stoichiometry: The Quantitative Aspect
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. To understand what happens when 2.50 grams of copper reacts with oxygen, we need to perform stoichiometric calculations to determine the mass of oxygen required and the mass of copper oxide produced.
Molar Mass
To perform stoichiometric calculations, we need the molar masses of the reactants and products:
- Copper (Cu): 63.55 g/mol
- Oxygen (O₂): 32.00 g/mol
- Copper(I) Oxide (Cu₂O): 143.10 g/mol
- Copper(II) Oxide (CuO): 79.55 g/mol
Step-by-Step Analysis
Let's consider the scenario where copper reacts to form Copper(II) Oxide (CuO).
Step 1: Convert Mass of Copper to Moles
To begin, convert the mass of copper (2.50 g) to moles using its molar mass:
Moles of Cu = Mass of Cu / Molar Mass of Cu
Moles of Cu = 2.50 g / 63.55 g/mol
Moles of Cu ≈ 0.0393 mol
Step 2: Determine the Moles of Oxygen Required
Using the balanced chemical equation for the formation of Copper(II) Oxide:
2Cu(s) + O₂(g) → 2CuO(s)
We see that 2 moles of copper react with 1 mole of oxygen. Therefore, the mole ratio of Cu to O₂ is 2:1.
Moles of O₂ = (Moles of Cu) / 2
Moles of O₂ = 0.0393 mol / 2
Moles of O₂ ≈ 0.0197 mol
Step 3: Calculate the Mass of Oxygen Required
Convert the moles of oxygen to grams using its molar mass:
Mass of O₂ = Moles of O₂ × Molar Mass of O₂
Mass of O₂ = 0.0197 mol × 32.00 g/mol
Mass of O₂ ≈ 0.630 g
Step 4: Calculate the Moles of Copper(II) Oxide Produced
From the balanced equation, 2 moles of copper produce 2 moles of Copper(II) Oxide. Thus, the mole ratio of Cu to CuO is 1:1.
Moles of CuO = Moles of Cu
Moles of CuO = 0.0393 mol
Step 5: Calculate the Mass of Copper(II) Oxide Produced
Convert the moles of Copper(II) Oxide to grams using its molar mass:
Mass of CuO = Moles of CuO × Molar Mass of CuO
Mass of CuO = 0.0393 mol × 79.55 g/mol
Mass of CuO ≈ 3.13 g
Summary for CuO Formation:
-
- 50 g of copper reacts with approximately 0.630 g of oxygen to produce about 3.13 g of Copper(II) Oxide.
If Copper(I) Oxide (Cu₂O) is Formed
Now, let's analyze the scenario where copper reacts to form Copper(I) Oxide (Cu₂O).
Step 1: Convert Mass of Copper to Moles
The moles of copper remain the same as before:
Moles of Cu = 0.0393 mol
Step 2: Determine the Moles of Oxygen Required
Using the balanced chemical equation for the formation of Copper(I) Oxide:
4Cu(s) + O₂(g) → 2Cu₂O(s)
We see that 4 moles of copper react with 1 mole of oxygen. Therefore, the mole ratio of Cu to O₂ is 4:1.
Moles of O₂ = (Moles of Cu) / 4
Moles of O₂ = 0.0393 mol / 4
Moles of O₂ ≈ 0.00983 mol
Step 3: Calculate the Mass of Oxygen Required
Convert the moles of oxygen to grams using its molar mass:
Mass of O₂ = Moles of O₂ × Molar Mass of O₂
Mass of O₂ = 0.00983 mol × 32.00 g/mol
Mass of O₂ ≈ 0.315 g
Step 4: Calculate the Moles of Copper(I) Oxide Produced
From the balanced equation, 4 moles of copper produce 2 moles of Copper(I) Oxide. Thus, the mole ratio of Cu to Cu₂O is 2:1.
Moles of Cu₂O = (Moles of Cu) / 2
Moles of Cu₂O = 0.0393 mol / 2
Moles of Cu₂O ≈ 0.0197 mol
Step 5: Calculate the Mass of Copper(I) Oxide Produced
Convert the moles of Copper(I) Oxide to grams using its molar mass:
Mass of Cu₂O = Moles of Cu₂O × Molar Mass of Cu₂O
Mass of Cu₂O = 0.0197 mol × 143.10 g/mol
Mass of Cu₂O ≈ 2.82 g
Summary for Cu₂O Formation:
-
- 50 g of copper reacts with approximately 0.315 g of oxygen to produce about 2.82 g of Copper(I) Oxide.
Factors Affecting the Reaction
Several factors influence the reaction between copper and oxygen, including:
-
Temperature: Higher temperatures generally accelerate the reaction rate. The energy required to overcome the activation energy barrier is more readily available at higher temperatures.
-
Oxygen Concentration: The availability of oxygen plays a crucial role in determining the type of copper oxide formed. Limited oxygen favors the formation of Copper(I) Oxide (Cu₂O), while an excess of oxygen favors Copper(II) Oxide (CuO).
-
Surface Area: The surface area of copper exposed to oxygen affects the reaction rate. A finely divided copper powder will react more rapidly than a solid block of copper due to the increased surface contact.
-
Presence of Catalysts: Certain substances can act as catalysts, accelerating the reaction without being consumed themselves. For instance, the presence of moisture or certain metal oxides can influence the reaction rate.
Observations and Visual Changes
When copper reacts with oxygen, several visual changes can be observed:
-
Color Change: The bright, reddish-orange color of metallic copper changes to either red (for Cu₂O) or black (for CuO), depending on the oxide formed.
-
Formation of a Layer: A layer of copper oxide forms on the surface of the copper. This layer can protect the underlying copper from further oxidation, a process known as passivation.
-
Heat Release: The reaction is exothermic, meaning it releases heat. This heat can be noticeable, especially when the reaction occurs rapidly.
Real-World Applications and Implications
The reaction between copper and oxygen has numerous real-world applications and implications:
-
Corrosion: The formation of copper oxide is a form of corrosion. While the oxide layer can protect the underlying metal, excessive oxidation can lead to structural weakening and failure.
-
Industrial Processes: Copper oxides are used in various industrial processes, including as catalysts, pigments, and precursors for other copper compounds.
-
Electronics: Copper is widely used in electronics due to its high conductivity. However, oxidation can reduce its conductivity, leading to performance issues.
-
Art and Architecture: The green patina that forms on copper roofs and statues is a result of the slow oxidation of copper, forming copper carbonates and sulfates.
Experimental Considerations
Conducting an experiment to observe the reaction between copper and oxygen requires careful consideration of several factors to ensure accurate and safe results.
Materials Required
- Copper wire or foil
- Bunsen burner or hot plate
- Crucible or heat-resistant container
- Balance
- Tongs
Procedure
- Weigh the Copper: Accurately weigh a piece of copper wire or foil using a balance. Record the initial mass.
- Heat the Copper: Place the copper in a crucible or heat-resistant container and heat it strongly using a Bunsen burner or hot plate. Ensure the copper is exposed to air.
- Observe Changes: Observe the color changes and the formation of the oxide layer on the copper surface.
- Cool and Weigh: Allow the copper oxide to cool to room temperature. Weigh the copper oxide and record the final mass.
- Calculations: Calculate the mass of oxygen that reacted with the copper and compare it to the theoretical values obtained from stoichiometric calculations.
Safety Precautions
- Wear Safety Goggles: Protect your eyes from potential splattering or fumes.
- Use Tongs: Handle hot objects with tongs to avoid burns.
- Work in a Well-Ventilated Area: Avoid inhaling fumes produced during the reaction.
- Handle Bunsen Burners with Care: Follow proper procedures for using a Bunsen burner.
Potential Sources of Error
- Incomplete Reaction: Ensure the copper is heated sufficiently to allow the reaction to proceed to completion.
- Loss of Oxide: Some copper oxide may be lost during the heating or cooling process.
- Impurities: Impurities in the copper sample can affect the accuracy of the results.
- Weighing Errors: Ensure accurate weighing of the copper and copper oxide.
Advanced Concepts
To delve deeper into the reaction between copper and oxygen, consider the following advanced concepts:
Thermodynamics
Thermodynamics provides insights into the energy changes associated with chemical reactions. The Gibbs free energy (ΔG) determines the spontaneity of a reaction. For the formation of copper oxides, the Gibbs free energy is negative at moderate temperatures, indicating that the reaction is spontaneous.
Kinetics
Chemical kinetics deals with the rates of chemical reactions. The rate of oxidation of copper depends on factors such as temperature, oxygen concentration, and the presence of catalysts. The Arrhenius equation describes the relationship between the reaction rate and temperature:
k = A × exp(-Ea / RT)
Where:
- k is the rate constant
- A is the pre-exponential factor
- Ea is the activation energy
- R is the gas constant
- T is the temperature
Electrochemical Considerations
The oxidation of copper can also be viewed from an electrochemical perspective. Copper can act as an anode in an electrochemical cell, losing electrons to form copper ions. This process is fundamental to understanding corrosion and electroplating.
Surface Chemistry
Surface chemistry plays a crucial role in the oxidation of copper. The adsorption of oxygen molecules on the copper surface is the first step in the reaction. The nature of the copper surface, including its crystal structure and surface defects, can influence the rate of oxidation.
FAQ Section
Q: What type of copper oxide is formed when copper is heated in air?
A: When copper is heated in air, primarily Copper(II) Oxide (CuO) is formed due to the abundance of oxygen. However, some Copper(I) Oxide (Cu₂O) may also form, especially in oxygen-deficient regions.
Q: Is the reaction between copper and oxygen reversible?
A: The reaction is not easily reversible under normal conditions. However, under specific conditions, such as high temperatures and reducing environments, copper oxide can be reduced back to copper.
Q: How does the color of copper oxide indicate its composition?
A: Copper(I) Oxide (Cu₂O) is typically red, while Copper(II) Oxide (CuO) is black. The color difference is due to the different electronic structures and energy levels of the two compounds.
Q: Can copper rust like iron?
A: Copper does not rust like iron. Rust is specifically the oxidation of iron, resulting in hydrated iron oxides. Copper forms copper oxides, which are chemically different from rust.
Q: How does the thickness of the oxide layer affect the underlying copper?
A: A thin layer of copper oxide can protect the underlying copper from further oxidation, a process known as passivation. However, a thick layer can lead to structural weakening and failure.
Conclusion
In conclusion, when 2.50 grams of copper reacts with oxygen, it undergoes a chemical transformation that results in the formation of copper oxide. The specific type of copper oxide formed (Cu₂O or CuO) depends on the reaction conditions, particularly the availability of oxygen. Stoichiometric calculations allow us to quantitatively determine the mass of oxygen required and the mass of copper oxide produced. Factors such as temperature, oxygen concentration, and surface area influence the reaction rate. The reaction has numerous real-world applications and implications, ranging from corrosion to industrial processes. Understanding the intricacies of this reaction provides valuable insights into the broader fields of chemistry, materials science, and engineering.
Latest Posts
Latest Posts
-
Enable Data Aggregation On Sites When Possible
Nov 06, 2025
-
Change The Scale Of The Worksheet To 85
Nov 06, 2025
-
Match Each Term To The Correct Definition
Nov 06, 2025
-
Label The Following Diagram With The Appropriate Terms
Nov 06, 2025
-
Unit 1 Progress Check Mcq Part B Answers
Nov 06, 2025
Related Post
Thank you for visiting our website which covers about When 2.50 G Of Copper Reacts With Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.