Write An Iupac Name For The Following Alkane/cycloalkane
arrobajuarez
Nov 26, 2025 · 10 min read
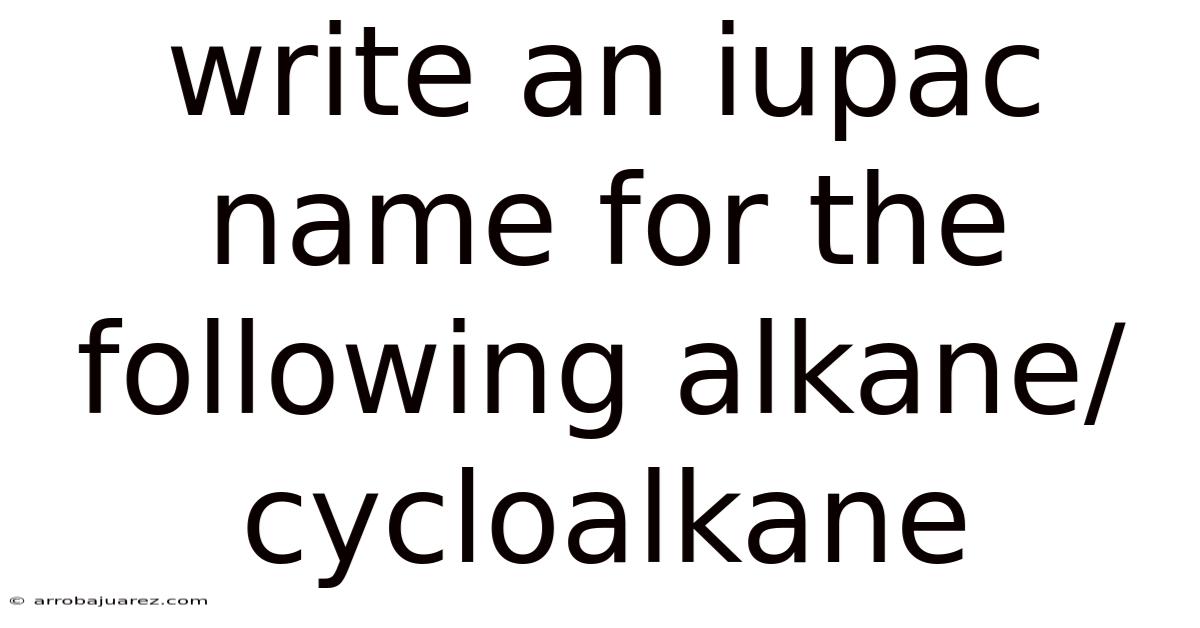
Table of Contents
Let's dive into the fascinating world of organic chemistry and unravel the mysteries of IUPAC nomenclature for alkanes and cycloalkanes. Naming organic compounds systematically is crucial for clear communication and understanding within the scientific community. The International Union of Pure and Applied Chemistry (IUPAC) has developed a standardized nomenclature system that provides a unique and unambiguous name for every organic compound. This guide will walk you through the fundamental rules and principles of IUPAC nomenclature for alkanes and cycloalkanes, equipping you with the knowledge to confidently name these fundamental organic molecules.
Understanding Alkanes and Cycloalkanes
Before we delve into the naming conventions, let's define what alkanes and cycloalkanes are:
-
Alkanes: These are saturated hydrocarbons, meaning they consist only of carbon and hydrogen atoms, with single bonds connecting them. They follow the general formula CₙH₂ₙ₊₂. Alkanes are acyclic, meaning they form open chains. Examples include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈).
-
Cycloalkanes: These are also saturated hydrocarbons, but their carbon atoms are arranged in a ring structure. They follow the general formula CₙH₂ₙ. Examples include cyclopropane (C₃H₆), cyclobutane (C₄H₈), and cyclohexane (C₆H₁₂).
Basic IUPAC Nomenclature Rules for Alkanes
The IUPAC nomenclature system for alkanes is based on a set of straightforward rules:
-
Identify the Parent Chain: This is the longest continuous chain of carbon atoms in the molecule. The name of the parent chain forms the root of the IUPAC name.
-
For example, if the longest chain contains six carbon atoms, the parent chain name is hexane. The names for the first ten continuous, unbranched alkanes are:
- 1: Methane
- 2: Ethane
- 3: Propane
- 4: Butane
- 5: Pentane
- 6: Hexane
- 7: Heptane
- 8: Octane
- 9: Nonane
- 10: Decane
-
-
Identify and Name the Substituents: Substituents are groups of atoms attached to the parent chain. Alkyl groups are the most common type of substituent in alkanes, and they are formed by removing one hydrogen atom from an alkane.
- For example, if a methyl group (CH₃) is attached to the parent chain, it is named as methyl. Other common alkyl substituents include ethyl (C₂H₅), propyl (C₃H₇), and butyl (C₄H₉).
-
Number the Parent Chain: Number the carbon atoms in the parent chain so that the substituents are attached to the carbon atoms with the lowest possible numbers. This is crucial for ensuring the name is unambiguous.
- For example, if a methyl group is attached to the second carbon atom of a hexane chain, the parent chain is numbered so that the methyl group is at position 2, not position 5.
-
Write the Name: Combine the substituent names, their positions on the parent chain, and the parent chain name into a single name.
- Substituents are listed in alphabetical order (ignoring prefixes like di-, tri-, etc.).
- Numbers are separated from each other by commas and from names by hyphens.
- If there are two or more identical substituents, use the prefixes di- (2), tri- (3), tetra- (4), penta- (5), and so on, to indicate the number of identical substituents.
IUPAC Nomenclature for Cycloalkanes
Naming cycloalkanes follows a similar set of rules, with a few modifications:
-
Identify the Parent Ring: The ring of carbon atoms is the parent structure. The name of the cycloalkane is formed by adding the prefix "cyclo-" to the name of the alkane with the same number of carbon atoms.
- For example, a six-membered ring is called cyclohexane.
-
Identify and Name the Substituents: As with alkanes, identify and name any substituents attached to the ring. Alkyl groups are common substituents.
-
Number the Ring: Number the carbon atoms in the ring so that the substituents are attached to the carbon atoms with the lowest possible numbers. If there are multiple substituents, number the ring to give the lowest possible set of numbers when the numbers are added together. If there is a choice, number the ring so that the substituent with the highest alphabetical priority has the lowest number.
-
Write the Name: Combine the substituent names, their positions on the ring, and the cycloalkane name into a single name.
- Substituents are listed in alphabetical order (ignoring prefixes like di-, tri-, etc.).
- Numbers are separated from each other by commas and from names by hyphens.
- If only one substituent is present, it is not necessary to indicate its position with a number (it is understood to be at position 1).
Examples and Practice
Let's work through some examples to solidify your understanding:
Example 1: A Simple Alkane
CH₃-CH₂-CH-CH₂-CH₂-CH₃ | CH₃
- Parent Chain: The longest continuous chain has six carbon atoms, so the parent chain is hexane.
- Substituent: There is a methyl group (CH₃) attached to the third carbon atom.
- Numbering: Number the chain from left to right so that the methyl group is at position 3.
- Name: 3-methylhexane
Example 2: A Branched Alkane
CH₃-CH-CH₂-CH-CH₃ | | CH₃ CH₂ | CH₃
- Parent Chain: The longest continuous chain has five carbon atoms, so the parent chain is pentane.
- Substituents: There is a methyl group (CH₃) attached to the second carbon atom and an ethyl group (CH₂CH₃) attached to the fourth carbon atom.
- Numbering: Number the chain from left to right.
- Name: 4-ethyl-2-methylpentane (ethyl comes before methyl alphabetically)
Example 3: A Simple Cycloalkane
A cyclohexane ring with a single methyl substituent.
- Parent Ring: The ring has six carbon atoms, so the parent ring is cyclohexane.
- Substituent: There is a methyl group (CH₃) attached to the ring.
- Numbering: Since there is only one substituent, numbering is not required.
- Name: methylcyclohexane
Example 4: A Cycloalkane with Multiple Substituents
A cyclohexane ring with a methyl group and an ethyl group.
- Parent Ring: The ring has six carbon atoms, so the parent ring is cyclohexane.
- Substituents: There is a methyl group (CH₃) and an ethyl group (CH₂CH₃) attached to the ring.
- Numbering: Number the ring starting at the ethyl group (because ethyl comes before methyl alphabetically) and proceed in the direction that gives the methyl group the lowest possible number. This results in the ethyl group being at position 1 and the methyl group being at position 2.
- Name: 1-ethyl-2-methylcyclohexane
Example 5: A Complex Alkane with Multiple Substituents
CH3 CH3
| |
CH3-CH-CH2-C-CH2-CH3
|
CH2-CH3
- Parent Chain: The longest continuous chain has 6 carbons (hexane).
- Substituents: There are two methyl groups and one ethyl group.
- Numbering: Number the chain to give the substituents the lowest possible numbers: 2,4,4
- Name: 4-ethyl-2,4-dimethylhexane
Example 6: A Cycloalkane with a Complex Substituent
A cyclohexane ring with an isopropyl group.
- Parent Ring: The ring has six carbon atoms, so the parent ring is cyclohexane.
- Substituent: The substituent is an isopropyl group (CH(CH₃)₂).
- Numbering: Not required since there is only one substituent.
- Name: isopropylcyclohexane
Advanced Considerations
While the basic rules cover most simple alkanes and cycloalkanes, there are some advanced considerations to keep in mind:
-
Complex Substituents: When a substituent is itself branched, it is named as a substituted alkyl group and enclosed in parentheses. For example, a substituent with a methyl group on the second carbon of a propyl group would be named 1-methylpropyl. The carbon atom attached directly to the parent chain is always given the number 1.
-
Alkenes and Alkynes: If the molecule contains double (alkenes) or triple (alkynes) bonds, the parent chain must include the double or triple bond, and the numbering must prioritize the double or triple bond, giving it the lowest possible number. The suffixes "-ene" and "-yne" are used to indicate the presence of double and triple bonds, respectively.
-
Functional Groups: When other functional groups (e.g., alcohols, ketones, carboxylic acids) are present, they take priority in naming. The parent chain must include the functional group, and the numbering must prioritize the functional group. These functional groups are indicated using specific suffixes or prefixes according to IUPAC rules.
-
Bicyclic and Polycyclic Compounds: Naming bicyclic and polycyclic compounds involves more complex rules, including identifying the bridgehead carbons (carbons shared by two or more rings) and using prefixes like "bicyclo-" and "tricyclo-" to indicate the number of rings.
Common Mistakes to Avoid
- Incorrectly Identifying the Parent Chain: Always look for the longest continuous chain, even if it is not drawn in a straight line.
- Incorrect Numbering: Ensure the substituents have the lowest possible numbers. If there is ambiguity, follow the alphabetical priority rule.
- Forgetting Alphabetical Order: List substituents in alphabetical order, ignoring prefixes like di-, tri-, etc.
- Not Using Prefixes Correctly: Use prefixes like di-, tri-, tetra- to indicate multiple identical substituents.
- Ignoring Cyclo- Prefix: Don't forget to add "cyclo-" to the name of cyclic alkanes.
Practice Problems
To test your understanding, try naming the following compounds:
- CH₃-CH₂-CH(CH₃)-CH₂-CH₂-CH₃
- CH₃-CH(CH₃)-CH(CH₃)-CH₃
- Cyclopentane with two methyl groups at positions 1 and 3.
- Cyclohexane with an ethyl group and a propyl group at positions 1 and 4, respectively.
- CH₃-CH₂-C(CH₃)₂-CH₂-CH₃
(Answers at the end of the article)
The Importance of IUPAC Nomenclature
The IUPAC nomenclature system is essential for several reasons:
- Unambiguous Communication: It provides a standardized way to name chemical compounds, eliminating confusion and ensuring that scientists worldwide understand exactly which compound is being discussed.
- Information Retrieval: IUPAC names are used in databases and scientific literature, allowing researchers to easily search for and retrieve information about specific compounds.
- Predicting Properties: The IUPAC name can provide clues about the structure and properties of a compound. For example, the presence of certain functional groups in the name indicates specific chemical behaviors.
- Legal and Regulatory Compliance: IUPAC names are often used in legal and regulatory contexts, such as in patents and safety data sheets (SDS).
Conclusion
Mastering IUPAC nomenclature for alkanes and cycloalkanes is a fundamental skill in organic chemistry. By understanding the basic rules and practicing consistently, you can confidently name these important organic molecules. Remember to always identify the parent chain or ring, name the substituents, number the chain or ring correctly, and write the name according to IUPAC conventions. As you continue your study of organic chemistry, you will encounter more complex molecules and functional groups, but the foundational principles learned here will serve you well. So, embrace the challenge, practice diligently, and unlock the power of chemical nomenclature!
FAQ
Q: What if there are two possible parent chains of equal length?
A: Choose the parent chain that has the greater number of substituents.
Q: How do I handle complex substituents?
A: Name the complex substituent as a substituted alkyl group, placing it in parentheses. The carbon atom of the substituent attached to the main chain is always numbered as 1.
Q: What if a cycloalkane is attached to an alkane chain?
A: If the number of carbon atoms in the ring is greater than or equal to the number of carbon atoms in the chain, the cycloalkane is the parent. If the chain is longer, the alkane is the parent, and the cycloalkane becomes a substituent (named as a cycloalkyl group).
Q: Are there exceptions to the IUPAC rules?
A: While IUPAC nomenclature aims to be comprehensive, some common names are still used, particularly for simpler compounds (e.g., isobutane instead of 2-methylpropane). However, using IUPAC names is always the most precise and unambiguous approach.
Q: Where can I find more resources for learning IUPAC nomenclature?
A: Numerous online resources, textbooks, and interactive tutorials are available. The IUPAC website itself provides detailed information and guidelines. Practice problems are essential for mastering the subject.
Answers to Practice Problems:
- 3-methylhexane
- 2,3-dimethylbutane
- 1,3-dimethylcyclopentane
- 1-ethyl-4-propylcyclohexane
- 3,3-dimethylpentane
Latest Posts
Latest Posts
-
Which Of The Following Elements Has The Largest Ionization Energy
Nov 26, 2025
-
Which Form Contains The Drivers Information
Nov 26, 2025
-
The Median Earnings For A 25 To 34
Nov 26, 2025
-
Charles Lackey Operates A Bakery In Idaho Falls
Nov 26, 2025
-
The Strategy Making Strategy Executing Process Is Shaped By
Nov 26, 2025
Related Post
Thank you for visiting our website which covers about Write An Iupac Name For The Following Alkane/cycloalkane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.