1 S 1 S 1 F
arrobajuarez
Nov 23, 2025 · 11 min read
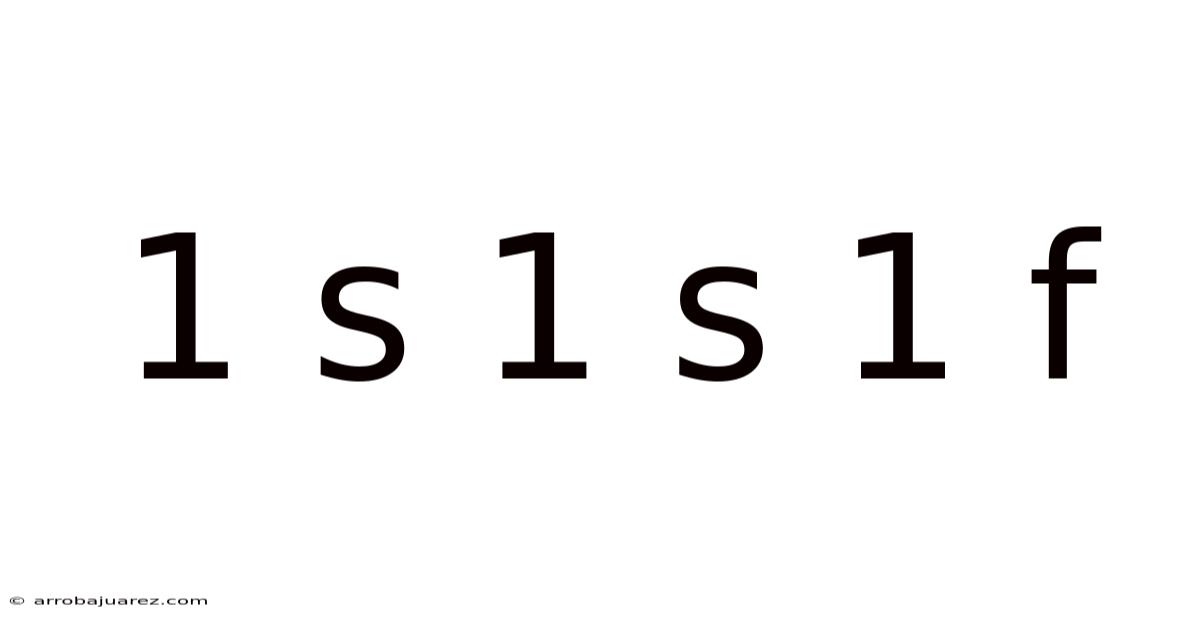
Table of Contents
Unveiling the Secrets of 1s 1s 1f: A Deep Dive into Electronic Configurations and Atomic Behavior
The notation "1s 1s 1f" might seem cryptic at first glance, but it unveils a fascinating glimpse into the world of electronic configurations and the fundamental principles governing atomic behavior. While the specific configuration presented (1s 1s 1f) is theoretically interesting and highlights potential violations of established rules, it serves as a powerful tool to understand how electrons arrange themselves within an atom, dictating its chemical properties and interactions. This comprehensive exploration will delve into the meaning behind this notation, the rules governing electron configurations, why 1s 1s 1f is typically not observed in ground-state atoms, and the broader implications for understanding the behavior of matter.
Understanding Electronic Configuration: A Foundation
Before we dissect the nuances of "1s 1s 1f," let's establish a solid foundation in the fundamentals of electronic configuration. Atoms, the building blocks of all matter, consist of a positively charged nucleus surrounded by negatively charged electrons. These electrons aren't simply orbiting the nucleus in random paths; instead, they occupy specific energy levels and regions of space called atomic orbitals.
- Principal Quantum Number (n): This number describes the energy level of an electron. Higher values of 'n' indicate higher energy levels and greater distance from the nucleus (n = 1, 2, 3, etc.). We often refer to these as electron shells.
- Azimuthal Quantum Number (l): Also known as the angular momentum or orbital shape quantum number, this dictates the shape of the electron's orbital. For a given 'n', 'l' can range from 0 to n-1. Each 'l' value corresponds to a specific subshell:
- l = 0: s orbital (spherical shape)
- l = 1: p orbital (dumbbell shape)
- l = 2: d orbital (more complex shape)
- l = 3: f orbital (even more complex shape)
- Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. For a given 'l', ml can range from -l to +l, including 0. This means:
- s orbital (l=0) has only one orientation (ml = 0).
- p orbital (l=1) has three orientations (ml = -1, 0, +1).
- d orbital (l=2) has five orientations (ml = -2, -1, 0, +1, +2).
- f orbital (l=3) has seven orientations (ml = -3, -2, -1, 0, +1, +2, +3).
- Spin Quantum Number (ms): This number describes the intrinsic angular momentum of an electron, which is quantized and referred to as spin. An electron behaves as if it is spinning, creating a magnetic dipole moment. The spin quantum number can be either +1/2 (spin up) or -1/2 (spin down).
These four quantum numbers (n, l, ml, ms) completely define the state of an electron in an atom.
Deciphering "1s 1s 1f": A Breakdown
Now, let's apply our knowledge to the notation "1s 1s 1f." This notation attempts to describe the electron configuration of an atom by specifying the occupied orbitals.
- 1s: This indicates that there are electrons occupying the s orbital of the first energy level (n=1). The s orbital in the first energy level can hold a maximum of two electrons, each with opposite spins (Pauli Exclusion Principle).
- 1s 1s: This suggests that the 1s orbital is doubly occupied, seemingly fulfilling the maximum capacity of this orbital. The repetition highlights that we are accounting for two electrons within that specific subshell.
- 1f: This implies the presence of an electron in the f orbital of the first energy level (n=1). This is where the problem arises.
Why 1s 1s 1f is Problematic: Violating the Rules
The electron configuration "1s 1s 1f" is highly unusual and, in most scenarios, violates the established rules governing electron distribution within an atom. Here's why:
-
The Azimuthal Quantum Number (l) Restriction: For the first energy level (n=1), the azimuthal quantum number (l) can only have one value: l = 0. This corresponds to the s orbital. There are no p, d, or f orbitals in the first energy level. Therefore, the designation "1f" is fundamentally impossible. The f orbitals only appear starting from the n=4 energy level.
-
Hund's Rule of Maximum Multiplicity: Hund's rule states that for a set of orbitals with the same energy (degenerate orbitals), electrons will individually occupy each orbital before doubling up in any one orbital. This maximizes the total spin angular momentum and minimizes electron-electron repulsion, leading to a more stable configuration. While this rule primarily applies to degenerate orbitals (like the three p orbitals or the five d orbitals), the presence of an electron in a higher energy (and non-existent for n=1) 1f orbital when the 1s orbital isn't fully occupied in a conventional sense goes against the principle of minimizing energy.
-
Energy Minimization Principle: Electrons tend to occupy the lowest energy levels available to them. In a typical atom, after filling the 1s orbital, electrons would move to the 2s orbital, then the 2p orbitals, and so on. The hypothetical presence of an electron in the 1f orbital would require significantly higher energy than occupying the 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, and 7s orbitals.
Hypothetical Scenarios: Exploring the Boundaries of Physics
While "1s 1s 1f" isn't a stable or typical configuration for ground-state atoms, it is useful to consider hypothetical scenarios where such a configuration might arise, even if only fleetingly or under extreme conditions. This exercise pushes the boundaries of our understanding and helps us appreciate the fundamental principles at play.
-
Extremely High Energy States: Imagine an atom subjected to an incredibly intense energy source, such as a powerful laser or a collision in a particle accelerator. In such extreme conditions, electrons might be forced into highly excited states, temporarily violating the usual energy level ordering. While the electron would rapidly decay to a lower energy state, for a fleeting moment, a configuration resembling "1s 1s 1f" might exist as a transient intermediate.
-
Exotic Atoms: Scientists are exploring the creation and properties of exotic atoms, where one or more of the constituent particles (electrons, protons, neutrons) are replaced by other particles with the same charge. For example, a muonic atom has one or more electrons replaced by muons, which are heavier versions of electrons. The heavier mass of the muon can significantly alter the energy levels and orbital sizes within the atom. In principle, with appropriate manipulation, the orbital filling rules we know may be altered.
-
Theoretical Modeling: Even if a configuration like "1s 1s 1f" is not directly observable, it can be explored through theoretical modeling and computational chemistry. These calculations can help us understand the energetic consequences of violating the usual electron configuration rules and provide insights into the limits of our current understanding of atomic structure.
The Aufbau Principle and Madelung's Rule: Predicting Electron Configurations
The Aufbau principle (from the German word "Aufbauen" meaning "to build up") provides a systematic way to predict the electron configuration of an atom by filling orbitals in order of increasing energy. Madelung's rule (also known as the (n+l) rule) provides a guideline for determining the order in which orbitals are filled:
- Orbitals are filled in order of increasing (n+l) value.
- If two orbitals have the same (n+l) value, the orbital with the lower 'n' value is filled first.
Using Madelung's rule, the predicted filling order is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Madelung's rule is an approximation, and there are exceptions, particularly for heavier elements where electron-electron interactions become more complex. However, it provides a useful starting point for understanding electron configurations. The existence of exceptions to Madelung's rule underscores the importance of considering the nuances of electronic configurations.
Consequences of Electron Configuration: Dictating Chemical Properties
The electron configuration of an atom has a profound influence on its chemical properties. The outermost electrons, known as valence electrons, are primarily responsible for how an atom interacts with other atoms to form chemical bonds.
- Reactivity: Atoms with incomplete valence shells tend to be more reactive, as they seek to gain, lose, or share electrons to achieve a stable electron configuration (usually a full outer shell, like the noble gases).
- Bonding: The type of chemical bond (ionic, covalent, metallic) that an atom forms depends on its electron configuration and electronegativity (the ability of an atom to attract electrons in a chemical bond).
- Oxidation State: The oxidation state of an atom reflects the number of electrons it has gained, lost, or shared in a chemical bond. This is directly related to its valence electron configuration.
Electron Configuration and the Periodic Table: A Powerful Relationship
The periodic table is organized based on the recurring patterns of chemical properties among the elements. These patterns arise directly from the similarities in the valence electron configurations of elements within the same group (vertical column) of the periodic table.
- Group 1 (Alkali Metals): All alkali metals have one valence electron in their outermost s orbital (e.g., Li: 1s²2s¹, Na: 1s²2s²2p⁶3s¹). This single valence electron is easily lost, making them highly reactive and forming +1 ions.
- Group 17 (Halogens): All halogens have seven valence electrons (e.g., F: 1s²2s²2p⁵, Cl: 1s²2s²2p⁶3s²3p⁵). They readily gain one electron to achieve a full outer shell, making them highly reactive and forming -1 ions.
- Group 18 (Noble Gases): Noble gases have a full outer shell of electrons (e.g., He: 1s², Ne: 1s²2s²2p⁶). This makes them exceptionally stable and unreactive (inert).
The periodic table, therefore, serves as a visual representation of the electron configurations of the elements and the resulting periodic trends in their chemical properties.
Beyond the Basics: Advanced Concepts in Electronic Configuration
While the Aufbau principle and Madelung's rule provide a good starting point, a more complete understanding of electron configurations requires considering more advanced concepts:
- Electron-Electron Interactions: The simple models often treat electrons as independent particles. In reality, electrons repel each other, and these interactions can significantly affect the energy levels of orbitals and the resulting electron configurations.
- Relativistic Effects: For heavier elements with high nuclear charges, the inner electrons move at relativistic speeds (close to the speed of light). These relativistic effects can alter the shapes and energies of orbitals, leading to deviations from the predictions of non-relativistic models.
- Term Symbols: Term symbols provide a more detailed description of the electronic state of an atom, taking into account the total angular momentum (both orbital and spin) of all the electrons. Term symbols are essential for understanding the spectra of atoms and their behavior in magnetic fields.
- Spectroscopic Notation: Spectroscopic notation uses term symbols to describe the electronic transitions that occur when atoms absorb or emit light. Analyzing atomic spectra provides valuable information about the energy levels and electron configurations of atoms.
The Significance of Understanding Electronic Configurations
Understanding electronic configurations is fundamental to numerous fields of science and technology:
- Chemistry: Predicting chemical reactivity, understanding bonding, and designing new molecules.
- Materials Science: Understanding the properties of materials (e.g., conductivity, magnetism, optical properties) and developing new materials with desired characteristics.
- Solid-State Physics: Describing the behavior of electrons in solids and understanding phenomena such as superconductivity.
- Spectroscopy: Analyzing the spectra of atoms and molecules to identify them and determine their properties.
- Quantum Computing: Manipulating the electronic states of atoms and ions to create quantum bits (qubits) for quantum computers.
FAQ: Addressing Common Questions about Electronic Configurations
-
Q: Can an electron have zero energy?
- A: No. According to quantum mechanics, an electron confined within an atom always possesses some non-zero kinetic energy. The lowest energy state is the ground state.
-
Q: What is the difference between electron configuration and orbital diagram?
- A: Electron configuration is a shorthand notation that describes the distribution of electrons among the various orbitals (e.g., 1s²2s²2p⁴). An orbital diagram is a more detailed representation that shows the individual orbitals as boxes or lines and the electrons as arrows, indicating their spin.
-
Q: Why do some elements have unexpected electron configurations?
- A: Deviations from the Aufbau principle often occur due to electron-electron interactions and the stability associated with half-filled or fully filled d or f subshells. For example, chromium (Cr) has the configuration [Ar] 3d⁵4s¹ instead of the predicted [Ar] 3d⁴4s².
-
Q: How does electronic configuration relate to ionization energy?
- A: Ionization energy is the energy required to remove an electron from an atom. Elements with weakly held valence electrons (e.g., alkali metals) have low ionization energies, while elements with nearly full valence shells (e.g., halogens) have high ionization energies.
Conclusion: The Enduring Importance of Electron Configurations
While the hypothetical "1s 1s 1f" configuration highlights the boundaries of established rules, the exercise emphasizes the importance of understanding the fundamental principles governing electron configurations. This knowledge is not merely an academic pursuit; it is the cornerstone of understanding the behavior of atoms and molecules, and it underpins countless applications in chemistry, materials science, and beyond. By delving into the intricacies of electron configurations, we gain a deeper appreciation for the elegant and complex nature of the quantum world and its profound impact on the world around us. Understanding electron configuration empowers us to predict and manipulate the properties of matter, paving the way for new discoveries and technological advancements.
Latest Posts
Latest Posts
-
Change The Page Orientation Of The Current Worksheet To Portrait
Nov 23, 2025
-
Which Term Includes A Word Part That Means Excessive
Nov 23, 2025
-
With Parent Company The Simulation Means
Nov 23, 2025
-
1 S 1 S 1 F
Nov 23, 2025
-
Choose The Best Definition Of Constitutional Isomers
Nov 23, 2025
Related Post
Thank you for visiting our website which covers about 1 S 1 S 1 F . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.