How Many Moles Of N2o4 Are In 76.3g N2o4
arrobajuarez
Nov 09, 2025 · 9 min read
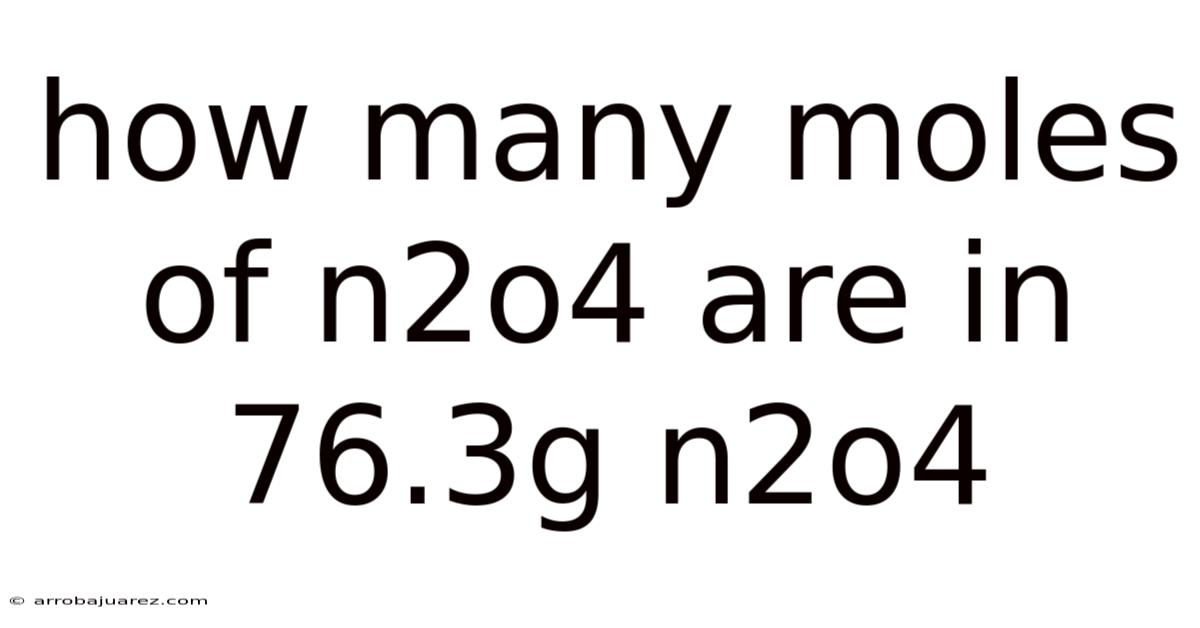
Table of Contents
The pursuit of quantitative understanding in chemistry often leads us to the concept of the mole, a fundamental unit that bridges the microscopic world of atoms and molecules with the macroscopic world we can measure. In this discussion, we will explore how to determine the number of moles in a given mass of a chemical substance, using dinitrogen tetroxide (N2O4) as our example. Specifically, we will calculate how many moles of N2O4 are present in 76.3 grams of N2O4.
Understanding the Mole Concept
The mole concept is central to stoichiometry, which deals with the quantitative relationships between reactants and products in chemical reactions. A mole is defined as the amount of a substance that contains as many entities (atoms, molecules, ions, etc.) as there are atoms in exactly 12 grams of carbon-12. This number, known as Avogadro's number, is approximately 6.022 x 10^23.
Why is the mole concept important?
- Relating Mass to Number of Particles: The mole provides a direct way to relate the mass of a substance to the number of atoms or molecules present.
- Stoichiometric Calculations: It enables accurate calculations in chemical reactions, ensuring that we know the precise amounts of reactants needed and products formed.
- Standardizing Measurements: The mole provides a standard unit for expressing the amount of a substance, facilitating consistent measurements across different experiments and studies.
Calculating Molar Mass of N2O4
Before we can convert grams of N2O4 to moles, we must first determine the molar mass of N2O4. The molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). To calculate the molar mass of N2O4, we add up the atomic masses of all the atoms in the molecule.
Step-by-Step Calculation of Molar Mass of N2O4:
-
Identify the Elements and their Quantities: N2O4 contains 2 nitrogen atoms (N) and 4 oxygen atoms (O).
-
Find the Atomic Masses: Look up the atomic masses of nitrogen and oxygen on the periodic table:
- Nitrogen (N): approximately 14.01 g/mol
- Oxygen (O): approximately 16.00 g/mol
-
Calculate the Total Mass: Multiply the atomic mass of each element by the number of atoms of that element in the molecule, then add these values together:
- (2 x Atomic mass of N) + (4 x Atomic mass of O)
- (2 x 14.01 g/mol) + (4 x 16.00 g/mol)
- 28.02 g/mol + 64.00 g/mol = 92.02 g/mol
Therefore, the molar mass of N2O4 is approximately 92.02 g/mol. This means that one mole of N2O4 weighs 92.02 grams.
Converting Grams to Moles
Now that we know the molar mass of N2O4, we can convert the given mass (76.3 g) into moles. The formula for converting mass to moles is:
Moles = Mass (g) / Molar Mass (g/mol)
Applying the Formula:
- Identify the Given Values:
- Mass of N2O4 = 76.3 g
- Molar mass of N2O4 = 92.02 g/mol
- Plug the Values into the Formula:
- Moles of N2O4 = 76.3 g / 92.02 g/mol
- Calculate the Result:
- Moles of N2O4 ≈ 0.829 moles
Therefore, there are approximately 0.829 moles of N2O4 in 76.3 grams of N2O4.
Practical Applications of Mole Calculations
Understanding how to convert between mass and moles is crucial in various chemical applications.
Examples:
- Chemical Synthesis: When synthesizing new compounds, chemists need to accurately measure reactants in molar quantities to ensure the reaction proceeds as expected and to maximize product yield.
- Analytical Chemistry: In analytical chemistry, determining the concentration of a substance often involves converting mass measurements to moles to perform quantitative analysis.
- Environmental Science: Environmental scientists use mole calculations to quantify pollutants in air, water, and soil, which helps in assessing environmental impact and developing remediation strategies.
- Pharmaceutical Industry: In the pharmaceutical industry, precise mole calculations are essential for formulating drugs and ensuring accurate dosages.
Common Mistakes and How to Avoid Them
When performing mole calculations, several common mistakes can lead to incorrect results. Being aware of these pitfalls can help you avoid them.
Common Mistakes:
- Using the Wrong Molar Mass: Ensure you are using the correct molar mass for the substance in question. Double-check the chemical formula and atomic masses.
- Incorrect Unit Conversions: Always use consistent units. If mass is given in kilograms, convert it to grams before using the formula.
- Rounding Errors: Avoid rounding intermediate values too early in the calculation, as this can affect the final result. Keep at least four significant figures throughout the calculation and round only at the end.
- Misunderstanding Chemical Formulas: Make sure you correctly interpret the chemical formula. For example, N2O4 has two nitrogen atoms and four oxygen atoms, not the other way around.
- Confusing Moles with Mass: Remember that moles and mass are different quantities. Moles represent the amount of a substance, while mass represents the quantity of matter.
Tips to Avoid Mistakes:
- Double-Check Your Work: Always review your calculations to ensure accuracy.
- Use a Calculator: A scientific calculator can help prevent arithmetic errors.
- Write Down Units: Including units in your calculations helps ensure that you are using the correct formulas and performing the correct conversions.
- Understand the Concepts: Make sure you have a solid understanding of the mole concept and molar mass calculations.
- Practice Regularly: The more you practice, the more comfortable you will become with these calculations and the less likely you are to make mistakes.
Advanced Concepts Related to Moles
Beyond basic mole calculations, several advanced concepts are essential for a deeper understanding of chemistry.
Key Concepts:
- Molarity: Molarity (M) is defined as the number of moles of solute per liter of solution. It is a common way to express the concentration of a solution.
- Molarity (M) = Moles of solute / Liters of solution
- Molality: Molality (m) is defined as the number of moles of solute per kilogram of solvent. It is used in situations where the temperature changes, as molality is independent of temperature.
- Molality (m) = Moles of solute / Kilograms of solvent
- Mole Fraction: Mole fraction (χ) is defined as the ratio of the number of moles of a component to the total number of moles of all components in a mixture.
- Mole fraction (χA) = Moles of component A / Total moles of all components
- Stoichiometry: Stoichiometry involves using mole ratios from balanced chemical equations to calculate the amounts of reactants and products in a chemical reaction.
- Limiting Reactant: In a chemical reaction, the limiting reactant is the reactant that is completely consumed first, thereby determining the maximum amount of product that can be formed.
- Percent Yield: Percent yield is the ratio of the actual yield (the amount of product obtained in a reaction) to the theoretical yield (the amount of product calculated from stoichiometry), expressed as a percentage.
- Percent Yield = (Actual Yield / Theoretical Yield) x 100%
Real-World Examples of N2O4
N2O4 is a colorless gas or liquid with significant applications, but it is also toxic and corrosive, requiring careful handling.
Uses and Applications:
- Rocket Propellant: N2O4 is used as an oxidizer in rocket propellants due to its ability to spontaneously ignite with various fuels.
- Chemical Synthesis: It is used as a reagent in various chemical syntheses, particularly in the production of explosives and other nitrogen-containing compounds.
- Laboratory Research: N2O4 is used in laboratory research for studying chemical reactions and properties of nitrogen oxides.
Safety Precautions:
- Toxicity: N2O4 is highly toxic and can cause severe respiratory and skin irritation. Exposure can lead to pulmonary edema and other serious health effects.
- Corrosiveness: It is a corrosive substance and can damage metals and other materials.
- Handling: N2O4 should be handled with extreme care in well-ventilated areas, using appropriate personal protective equipment (PPE), such as gloves, goggles, and respirators.
- Storage: It should be stored in tightly sealed containers in a cool, dry place away from combustible materials and incompatible substances.
The Importance of Significant Figures
In scientific calculations, significant figures are crucial for expressing the precision of measurements and calculations. When performing mole calculations, it is important to follow the rules for significant figures to ensure that your answer accurately reflects the precision of the given values.
Rules for Significant Figures:
- Non-zero digits are always significant. For example, 123.45 has five significant figures.
- Zeros between non-zero digits are significant. For example, 1002.05 has six significant figures.
- Leading zeros are not significant. For example, 0.0025 has two significant figures.
- Trailing zeros in a number containing a decimal point are significant. For example, 12.230 has five significant figures.
- Trailing zeros in a number not containing a decimal point may or may not be significant. To avoid ambiguity, use scientific notation. For example, 1200 can be written as 1.2 x 10^3 (two significant figures), 1.20 x 10^3 (three significant figures), or 1.200 x 10^3 (four significant figures).
Applying Significant Figures in Mole Calculations:
- When adding or subtracting numbers, the result should have the same number of decimal places as the number with the fewest decimal places.
- When multiplying or dividing numbers, the result should have the same number of significant figures as the number with the fewest significant figures.
In our example, we had 76.3 g of N2O4 (three significant figures) and a molar mass of 92.02 g/mol (four significant figures). Therefore, our final answer should be rounded to three significant figures:
Moles of N2O4 ≈ 0.829 moles
Practical Examples and Exercises
To solidify your understanding of mole calculations, let's work through some additional examples and exercises.
Example 1:
How many moles are present in 150.0 grams of sodium chloride (NaCl)?
- Find the Molar Mass of NaCl:
- Na (Sodium): 22.99 g/mol
- Cl (Chlorine): 35.45 g/mol
- Molar Mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
- Convert Grams to Moles:
- Moles of NaCl = 150.0 g / 58.44 g/mol = 2.567 moles
Therefore, there are 2.567 moles of NaCl in 150.0 grams.
Example 2:
What is the mass of 0.500 moles of glucose (C6H12O6)?
- Find the Molar Mass of C6H12O6:
- C (Carbon): 12.01 g/mol
- H (Hydrogen): 1.01 g/mol
- O (Oxygen): 16.00 g/mol
- Molar Mass of C6H12O6 = (6 x 12.01 g/mol) + (12 x 1.01 g/mol) + (6 x 16.00 g/mol) = 180.18 g/mol
- Convert Moles to Grams:
- Mass of C6H12O6 = 0.500 mol x 180.18 g/mol = 90.09 g
Therefore, 0.500 moles of glucose has a mass of 90.09 grams.
Conclusion
The ability to convert between mass and moles is a fundamental skill in chemistry, essential for performing stoichiometric calculations, understanding chemical reactions, and working with chemical substances. By understanding the mole concept, calculating molar masses, and applying the appropriate formulas, you can accurately determine the number of moles in a given mass of any substance. This knowledge is not only crucial for academic success but also for practical applications in various fields, including chemical synthesis, analytical chemistry, environmental science, and the pharmaceutical industry. Remember to pay attention to significant figures, avoid common mistakes, and practice regularly to master these essential skills.
Latest Posts
Related Post
Thank you for visiting our website which covers about How Many Moles Of N2o4 Are In 76.3g N2o4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.